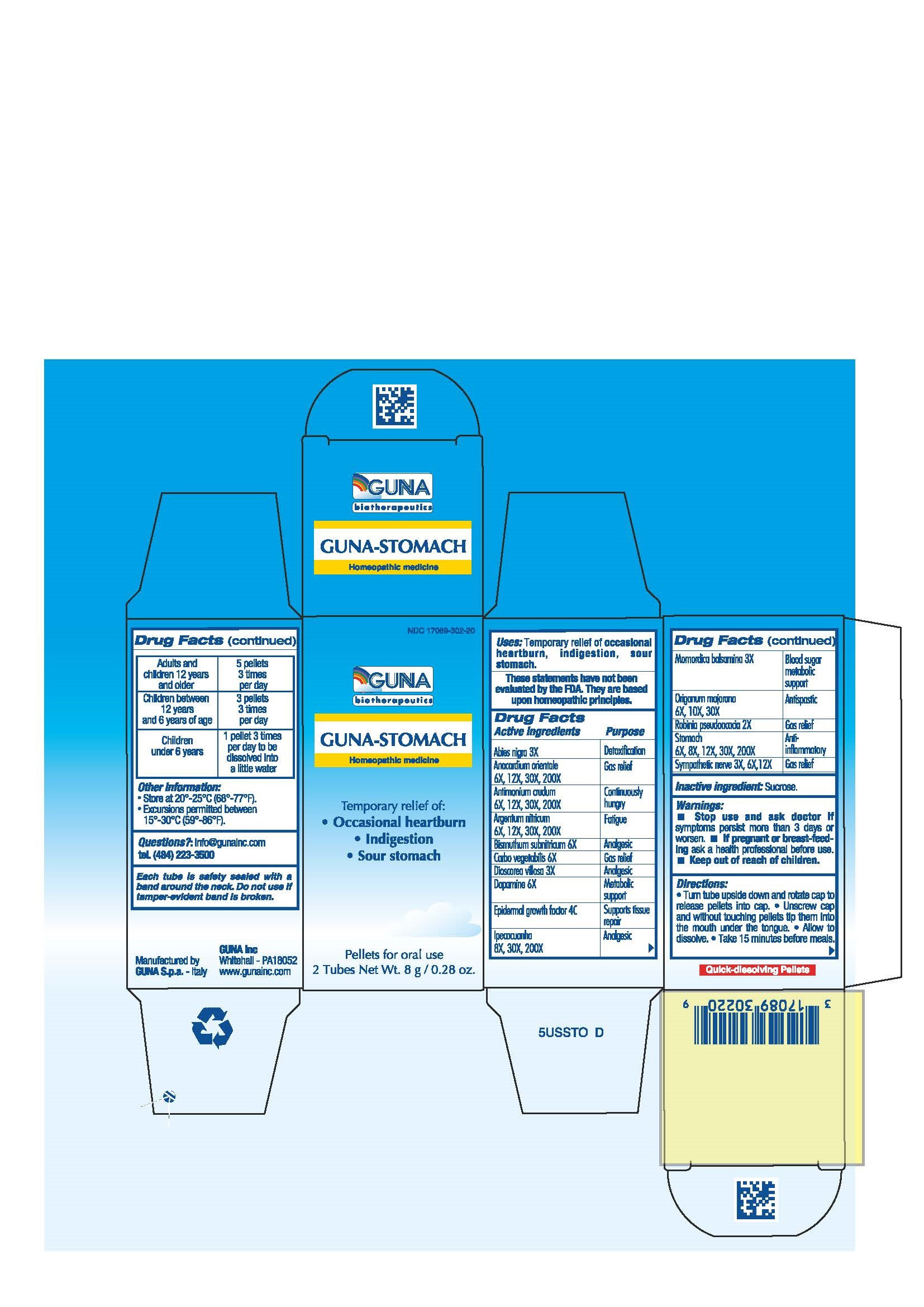
| NDC | 17089-302-20 |
| Set ID | 7805055c-e5f6-4d1d-9449-a5dce8a066eb |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Guna spa |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENTS/PURPOSE
ABIES NIGRA 3X DETOXIFICATION
ANACARDIUM ORIENTALE 6X, 12X, 30X, 200X GAS RELIEF
ANTIMONIUM CRUDUM 6X, 12X, 30X, 200X CONTINUOUSLY HUNGRY
ARGENTUM NITRICUM 6X, 12X, 30X, 200X FATIGUE
BISMUTHUM SUBNITRICUM 6X ANALGESIC
CARBO VEGETALIS 6X GAS RELIEF
DIOSCOREA VILLOSA 3X ANALGESIC
DOPAMINE 6X METABOLIC SUPPORT
EPIDERMAL GROWTH FACTOR 4C SUPPORT TISSUE REPAIR
IPECACUANHA 8X, 30X, 200X ANALGESIC
MOMORDICA BALSAMINA 3X BLOOD SUGAR METABOLIC SUPPORT
ORIGANUM MAJORANA 6X, 10X, 30X ANTISPASTIC
ROBINIA PSEUDOACACIA 2X GAS RELIEF
STOMACH 6X, 8X, 12X, 30X, 200X ANTI-INFLAMMATORY
SYMPATHETIC NERVE 3X, 6X, 12X GAS RELIEF - USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-STOMACH
activated charcoal - antimony trisulfide - bismuth subnitrate - dioscorea villosa root - dopamine - human epidermal growth factor - ipecac - oregano - picea mariana resin - robinia pseudoacacia bark - semecarpus anacardium juice - silver nitrate - sus scrofa stomach - sus scrofa sympathetic nerve - momordica balsamina immature fruit - pelletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-302 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PICEA MARIANA RESIN (UNII: 71AOV0W131) (PICEA MARIANA RESIN - UNII:71AOV0W131) PICEA MARIANA RESIN 3 [hp_X] in 4 g SEMECARPUS ANACARDIUM JUICE (UNII: Y0F0BU8RDU) (SEMECARPUS ANACARDIUM JUICE - UNII:Y0F0BU8RDU) SEMECARPUS ANACARDIUM JUICE 30 [hp_X] in 4 g ANTIMONY TRISULFIDE (UNII: F79059A38U) (ANTIMONY CATION (3+) - UNII:069647RPT5) ANTIMONY TRISULFIDE 30 [hp_X] in 4 g SILVER NITRATE (UNII: 95IT3W8JZE) (SILVER CATION - UNII:57N7B0K90A) SILVER NITRATE 30 [hp_X] in 4 g BISMUTH SUBNITRATE (UNII: H19J064BA5) (BISMUTH CATION - UNII:ZS9CD1I8YE) BISMUTH SUBNITRATE 6 [hp_X] in 4 g ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 6 [hp_X] in 4 g DIOSCOREA VILLOSA ROOT (UNII: IWY3IWX2G8) (DIOSCOREA VILLOSA ROOT - UNII:IWY3IWX2G8) DIOSCOREA VILLOSA ROOT 3 [hp_X] in 4 g DOPAMINE (UNII: VTD58H1Z2X) (DOPAMINE - UNII:VTD58H1Z2X) DOPAMINE 6 [hp_X] in 4 g HUMAN EPIDERMAL GROWTH FACTOR (UNII: TZK30RF92W) (HUMAN EPIDERMAL GROWTH FACTOR - UNII:TZK30RF92W) HUMAN EPIDERMAL GROWTH FACTOR 4 [hp_C] in 4 g IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 30 [hp_X] in 4 g MOMORDICA BALSAMINA IMMATURE FRUIT (UNII: WUW1665V10) (MOMORDICA BALSAMINA IMMATURE FRUIT - UNII:WUW1665V10) MOMORDICA BALSAMINA IMMATURE FRUIT 3 [hp_X] in 4 g OREGANO (UNII: 0E5AT8T16U) (OREGANO - UNII:0E5AT8T16U) OREGANO 10 [hp_X] in 4 g ROBINIA PSEUDOACACIA BARK (UNII: 7TPC058OWY) (ROBINIA PSEUDOACACIA BARK - UNII:7TPC058OWY) ROBINIA PSEUDOACACIA BARK 2 [hp_X] in 4 g SUS SCROFA STOMACH (UNII: T0920P9Z9A) (SUS SCROFA STOMACH - UNII:T0920P9Z9A) SUS SCROFA STOMACH 30 [hp_X] in 4 g SUS SCROFA SYMPATHETIC NERVE (UNII: H6L0IFR3FE) (SUS SCROFA SYMPATHETIC NERVE - UNII:H6L0IFR3FE) SUS SCROFA SYMPATHETIC NERVE 6 [hp_X] in 4 g Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) 3.5 g in 4 g Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-302-20 2 in 1 BOX 05/15/2018 1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/15/2018 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-302)