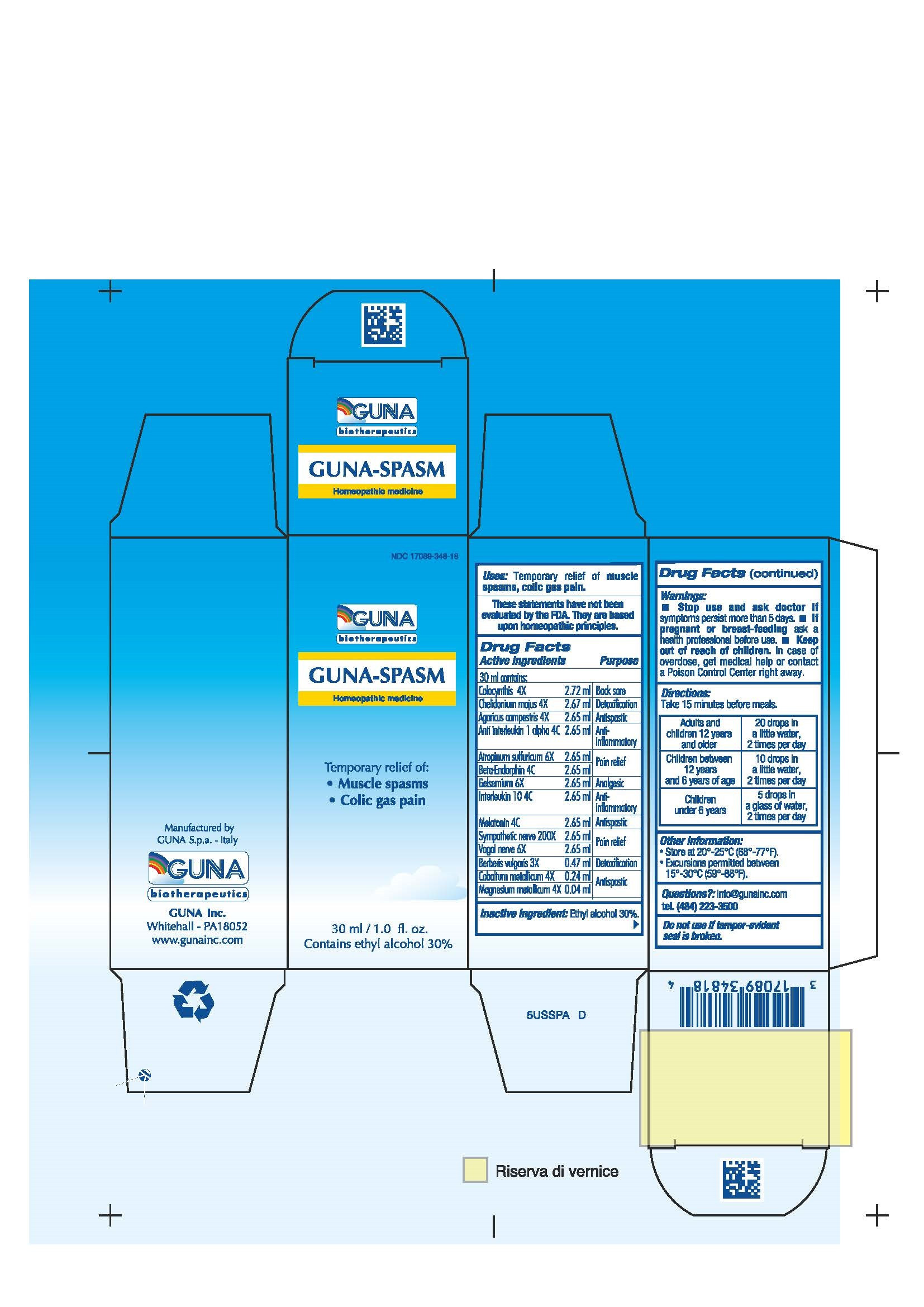
| NDC | 17089-348-18 |
| Set ID | 9d46b4d1-c8e8-40a3-8a32-1cc3d8f602ed |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Guna spa |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENTS/PURPOSE
AGARICUS CAMPESTRIS 4X ANTISPASTIC
ANTI INTERLEUKIN 1 ALPHA 4C ANTI-INFLAMMATORY
ATROPINUM SULFURICUM 6X PAIN RELIEF
BERBERIS VULGARIS 3X DETOXIFICATION
BETA-ENDORPHIN 4C PAIN RELIEF
CHELIDONIUM MAJUS 4X DETOXIFICATION
COBALTUM METALLICUM 4X ANTISPASTIC
COLOCYNTHIS 4X BACK SORE
GELSEMIUM 6X ANALGESIC
INTERLEUKIN 10 4C ANTI-INFLAMMATORY
MAGNESIUM METALLICUM 4X ANTISPASTIC
MELATONIN 4C ANTISPASTIC
SYMPATHETIC NERVE 200X PAIN RELIEF
VAGAL NERVE 6X PAIN RELIEF - USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-SPASM
atropine sulfate - berberis vulgaris root bark - chelidonium majus - cobalt - cultivated mushroom - gelsemium sempervirens root - interleukin-10 - magnesium - melatonin - metenkefalin - sus scrofa sympathetic nerve - sus scrofa vagus nerve - watermelon - anti-interleukin-1.alpha. immunoglobulin g rabbit - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-348 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CULTIVATED MUSHROOM (UNII: 54C8E6W6JY) (CULTIVATED MUSHROOM - UNII:54C8E6W6JY) CULTIVATED MUSHROOM 4 [hp_X] in 30 mL ANTI-INTERLEUKIN-1.ALPHA. IMMUNOGLOBULIN G RABBIT (UNII: ML4QRZ1HCL) (ANTI-INTERLEUKIN-1.ALPHA. IMMUNOGLOBULIN G RABBIT - UNII:ML4QRZ1HCL) ANTI-INTERLEUKIN-1.ALPHA. IMMUNOGLOBULIN G RABBIT 4 [hp_C] in 30 mL ATROPINE SULFATE (UNII: 03J5ZE7KA5) (ATROPINE - UNII:7C0697DR9I) ATROPINE SULFATE 6 [hp_X] in 30 mL BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 3 [hp_X] in 30 mL METENKEFALIN (UNII: 9JEZ9OD3AS) (METENKEFALIN - UNII:9JEZ9OD3AS) METENKEFALIN 4 [hp_C] in 30 mL CHELIDONIUM MAJUS (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS - UNII:7E889U5RNN) CHELIDONIUM MAJUS 4 [hp_X] in 30 mL COBALT (UNII: 3G0H8C9362) (COBALT - UNII:3G0H8C9362) COBALT 4 [hp_X] in 30 mL WATERMELON (UNII: 231473QB6R) (WATERMELON - UNII:231473QB6R) WATERMELON 4 [hp_X] in 30 mL GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 6 [hp_X] in 30 mL INTERLEUKIN-10 (UNII: 9SC4O216V9) (INTERLEUKIN-10 - UNII:9SC4O216V9) INTERLEUKIN-10 4 [hp_C] in 30 mL MAGNESIUM (UNII: I38ZP9992A) (MAGNESIUM - UNII:I38ZP9992A) MAGNESIUM 4 [hp_X] in 30 mL MELATONIN (UNII: JL5DK93RCL) (MELATONIN - UNII:JL5DK93RCL) MELATONIN 4 [hp_C] in 30 mL SUS SCROFA SYMPATHETIC NERVE (UNII: H6L0IFR3FE) (SUS SCROFA SYMPATHETIC NERVE - UNII:H6L0IFR3FE) SUS SCROFA SYMPATHETIC NERVE 200 [hp_X] in 30 mL SUS SCROFA VAGUS NERVE (UNII: 5AZZ2GHR69) (SUS SCROFA VAGUS NERVE - UNII:5AZZ2GHR69) SUS SCROFA VAGUS NERVE 6 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 9 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-348-18 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/16/2008 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-348)