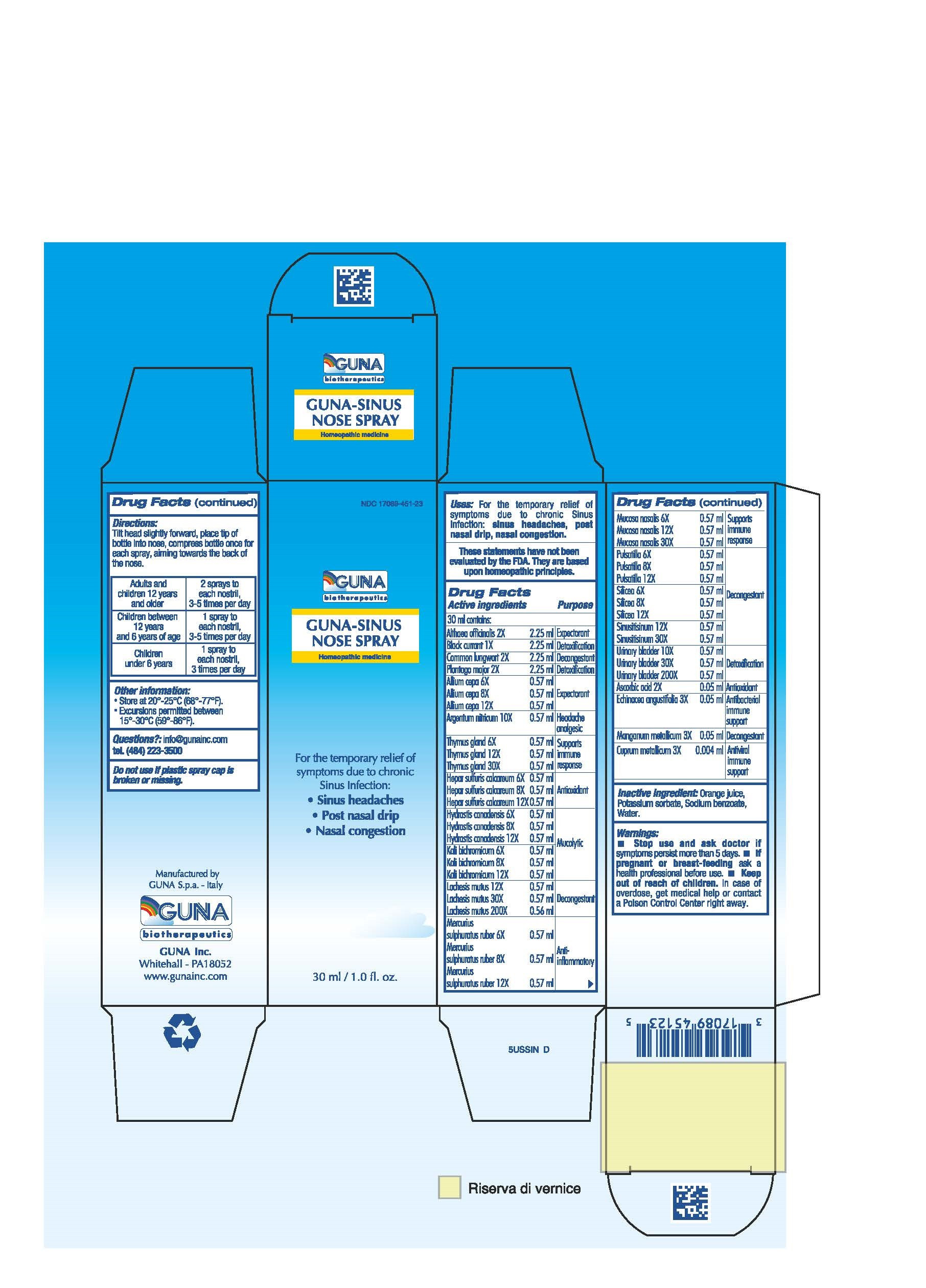
| NDC | 17089-451-23 |
| Set ID | 36fef678-17c6-4d2a-a0f0-ddb298611f25 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Guna spa |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENTS/PURPOSE
ALIUM CEPA 6X, 8X, 12X EXPECTORANT
ALTHEA OFFICINALIS 2X EXPECTORANT
ARGENTUM NITRICUM 10X HEADACHE ANALGESIC
ASCORBIC ACID 2X ANTIOXIDANT
BLACK CURRANT 1X DETOXIFICATION
COMMON LUNGWORT 2X DECONGESTANT
CUPRUM METALLICUM 3X ANTIVIRAL IMMUNE SUPPORT
ECHINACEA ANGUSTIFOLIA 3X ANTIBACTERIAL IMMUNE SUPPORT
HEPAR SULFURIS CALCAREUM 6X, 8X, 12X ANTIOXIDANT
HYDRASTIS CANADENSIS 6X, 8X, 12X MUCOLYTIC
KALI BICHROMICUM 6X, 8X, 12X MUCOLYTIC
LACHESIS MUTUS 12X, 30X, 200X DECONGESTANT
MANGANUM METALLICUM 3X DECONGESTANT
MERCURIUS SULPHURATUS RUBER 6X, 8X, 12X ANTI-INFLAMMATORY
MUCOSA NASALIS 6X, 12X, 30X SUPPORTS IMMUNE RESPONSE
PLANTAGO MAJOR 2X DETOXIFICATION
PULSATILLA 6X, 8X, 12X DECONGESTANT
SILICEA 6X, 8X, 12X DECONGESTANT
SINUSITISINUM 12X, 30X DECONGESTANT
THYMUS GLAND 6X, 12X, 30X SUPPORTS IMMUNE RESPONSE
URINARY BLADDER 10X, 30X, 200X DETOXIFICATION - USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-SINUS NOSE
althaea officinalis leaf - ascorbic acid - black currant - calcium sulfide - copper - echinacea angustifolia - goldenseal - lachesis muta venom - lobaria pulmonaria - manganese - mercuric sulfide - onion - plantago major - potassium carbonate - pulsatilla vulgaris - silicon dioxide - silver nitrate - sinusitisinum - sus scrofa nasal mucosa - sus scrofa thymus - sus scrofa urinary bladder - sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-451 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 8 [hp_X] in 30 mL ALTHAEA OFFICINALIS LEAF (UNII: E2QQV92338) (ALTHAEA OFFICINALIS LEAF - UNII:E2QQV92338) ALTHAEA OFFICINALIS LEAF 2 [hp_X] in 30 mL SILVER NITRATE (UNII: 95IT3W8JZE) (SILVER CATION - UNII:57N7B0K90A) SILVER NITRATE 10 [hp_X] in 30 mL ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 2 [hp_X] in 30 mL BLACK CURRANT (UNII: 9755T40D11) (BLACK CURRANT - UNII:9755T40D11) BLACK CURRANT 1 [hp_X] in 30 mL LOBARIA PULMONARIA (UNII: D1YM0P5Z2T) (LOBARIA PULMONARIA - UNII:D1YM0P5Z2T) LOBARIA PULMONARIA 2 [hp_X] in 30 mL COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 3 [hp_X] in 30 mL ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 3 [hp_X] in 30 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 8 [hp_X] in 30 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 8 [hp_X] in 30 mL POTASSIUM DICHROMATE (UNII: T4423S18FM) (DICHROMATE ION - UNII:9LKY4BFN2V) POTASSIUM DICHROMATE 8 [hp_X] in 30 mL LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 30 [hp_X] in 30 mL MANGANESE (UNII: 42Z2K6ZL8P) (MANGANESE - UNII:42Z2K6ZL8P) MANGANESE 3 [hp_X] in 30 mL MERCURIC SULFIDE (UNII: ZI0T668SF1) (MERCURIC CATION - UNII:ED30FJ8Y42) MERCURIC SULFIDE 8 [hp_X] in 30 mL SUS SCROFA NASAL MUCOSA (UNII: ID3Z1X61WY) (SUS SCROFA NASAL MUCOSA - UNII:ID3Z1X61WY) SUS SCROFA NASAL MUCOSA 12 [hp_X] in 30 mL PLANTAGO MAJOR (UNII: W2469WNO6U) (PLANTAGO MAJOR - UNII:W2469WNO6U) PLANTAGO MAJOR 2 [hp_X] in 30 mL PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 8 [hp_X] in 30 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 8 [hp_X] in 30 mL SINUSITISINUM (UNII: B575563DM5) (SINUSITISINUM - UNII:B575563DM5) SINUSITISINUM 30 [hp_X] in 30 mL SUS SCROFA THYMUS (UNII: 7B69B0BD62) (SUS SCROFA THYMUS - UNII:7B69B0BD62) SUS SCROFA THYMUS 12 [hp_X] in 30 mL SUS SCROFA URINARY BLADDER (UNII: 3G7U72W8DA) (SUS SCROFA URINARY BLADDER - UNII:3G7U72W8DA) SUS SCROFA URINARY BLADDER 30 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ORANGE JUICE (UNII: 5A9KE2L9L3) 0.6 mL in 30 mL POTASSIUM SORBATE (UNII: 1VPU26JZZ4) 0.03 mL in 30 mL WATER (UNII: 059QF0KO0R) 15.9 mL in 30 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 0.03 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-451-23 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/27/2010 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-451)