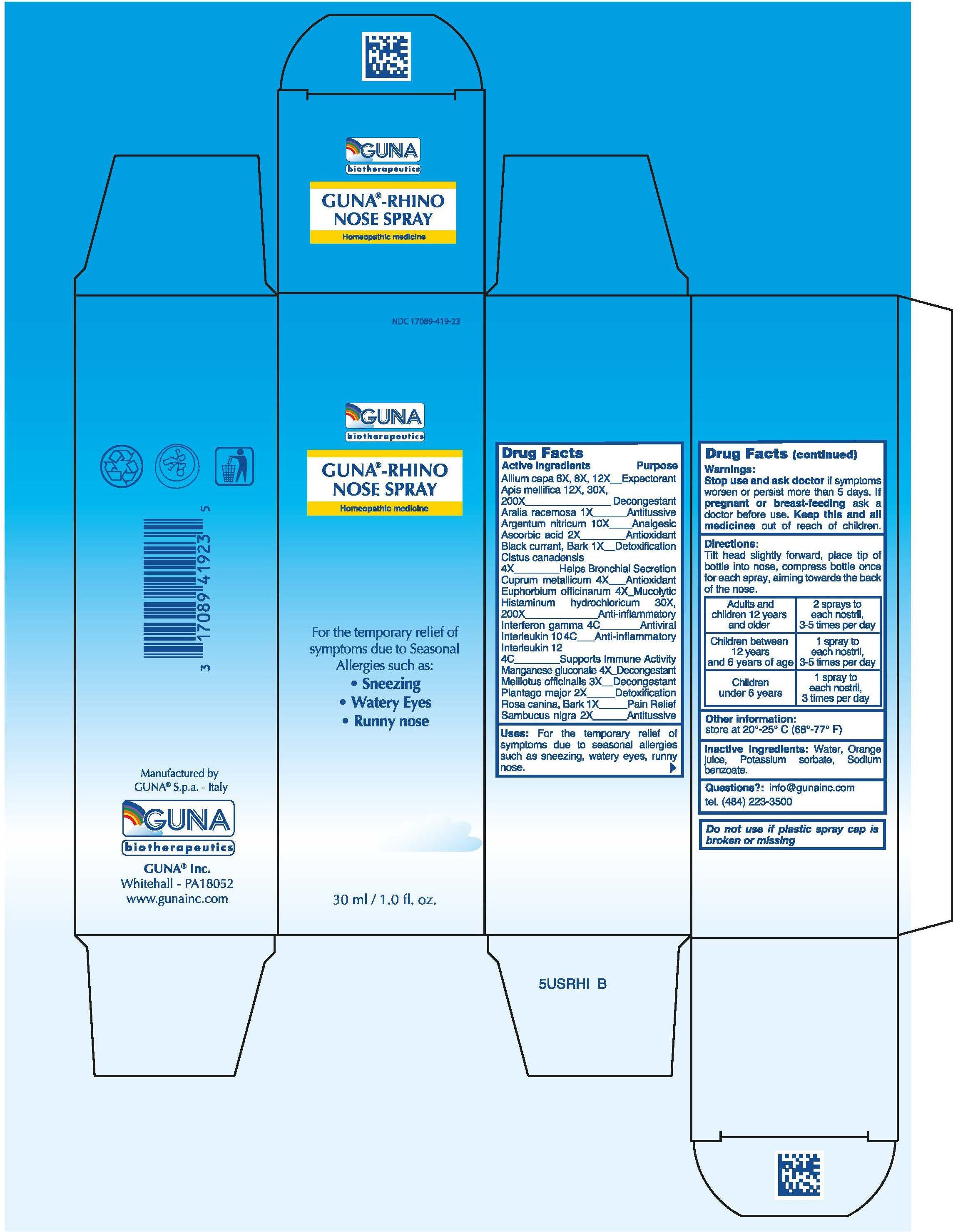
| NDC | 17089-419-23 |
| Set ID | 5baeb698-1735-4586-9984-2a2cb0da871a |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Guna spa |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENTS/PURPOSE
ALIUM CEPA 6X, 8X, 12X EXPECTORANT
APIS MELLIFICA 12x, 30X, 200X DECONGESTANT
ARALIA RACEMOSA 1X ANTITUSSIVE
ARGENTUM NITRICUM 10X ANALGESIC
ASCORBIC ACID 2X ANTIOXIDANT
BLACK CURRANT, BARK 1X DETOXIFICATION
CISTUS CANADENSIS 4X HELPS BRONCHIAL SECRETION
CUPRUM METALLICUM 4X ANTIOXIDANT
EUPHORBIUM OFFICINARUM 4X MUCOLYTIC
HISTAMINUM HYDROCHLORICUM 30X, 200X ANTI-INFLAMMATORY
INTERFERON GAMMA 4C ANTIVIRAL
INTERLEUKIN 10 4C ANTI-INFLAMMATORY
INTERLEUKIN 12 4C SUPPORTS IMMUNE ACTIVITY
MANGANESE GLUCONATE 4X DECONGESTANT
MELILOTUS OFFICINALIS 3X DECONGESTANT
PLANTAGO MAJOR 2X DETOXIFICATION
ROSA CANINA, BARK 1X PAIN RELIEF
SAMBUCUS NIGRA 2X ANTITUSSIVE - USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-RHINO NOSE
apis mellifera - aralia racemosa root - ascorbic acid - black currant - copper - euphorbia resinifera resin - european elderberry - helianthemum canadense - histamine dihydrochloride - human interleukin 12 - human interleukin-10 (nonglycosylated) - interferon gamma-1b - manganese gluconate - melilotus - onion - plantago major - rosa canina fruit - silver nitrate - sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-419 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 8 [hp_X] in 30 mL APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 12 [hp_X] in 30 mL ARALIA RACEMOSA ROOT (UNII: T90W4582DU) (ARALIA RACEMOSA ROOT - UNII:T90W4582DU) ARALIA RACEMOSA ROOT 1 [hp_X] in 30 mL SILVER NITRATE (UNII: 95IT3W8JZE) (SILVER CATION - UNII:57N7B0K90A) SILVER NITRATE 10 [hp_X] in 30 mL ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 2 [hp_X] in 30 mL BLACK CURRANT (UNII: 9755T40D11) (BLACK CURRANT - UNII:9755T40D11) BLACK CURRANT 1 [hp_X] in 30 mL HELIANTHEMUM CANADENSE (UNII: 46G3W789Q3) (HELIANTHEMUM CANADENSE - UNII:46G3W789Q3) HELIANTHEMUM CANADENSE 4 [hp_X] in 30 mL COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 4 [hp_X] in 30 mL EUPHORBIA RESINIFERA RESIN (UNII: 1TI1O9028K) (EUPHORBIA RESINIFERA RESIN - UNII:1TI1O9028K) EUPHORBIA RESINIFERA RESIN 4 [hp_X] in 30 mL HISTAMINE DIHYDROCHLORIDE (UNII: 3POA0Q644U) (HISTAMINE - UNII:820484N8I3) HISTAMINE DIHYDROCHLORIDE 200 [hp_X] in 30 mL INTERFERON GAMMA-1B (UNII: 21K6M2I7AG) (INTERFERON GAMMA-1B - UNII:21K6M2I7AG) INTERFERON GAMMA-1B 4 [hp_C] in 30 mL INTERLEUKIN-10 (UNII: 9SC4O216V9) (INTERLEUKIN-10 - UNII:9SC4O216V9) INTERLEUKIN-10 4 [hp_C] in 30 mL HUMAN INTERLEUKIN 12 (UNII: 7B590791ER) (HUMAN INTERLEUKIN 12 - UNII:7B590791ER) HUMAN INTERLEUKIN 12 4 [hp_C] in 30 mL MANGANESE GLUCONATE (UNII: 9YY2F980SV) (MANGANESE CATION (2+) - UNII:H6EP7W5457) MANGANESE GLUCONATE 4 [hp_X] in 30 mL MELILOTUS (UNII: F22I9R6Q0X) (MELILOTUS - UNII:F22I9R6Q0X) MELILOTUS 3 [hp_X] in 30 mL PLANTAGO MAJOR (UNII: W2469WNO6U) (PLANTAGO MAJOR - UNII:W2469WNO6U) PLANTAGO MAJOR 2 [hp_X] in 30 mL ROSA CANINA FRUIT (UNII: 3TNW8D08V3) (ROSA CANINA FRUIT - UNII:3TNW8D08V3) ROSA CANINA FRUIT 1 [hp_X] in 30 mL EUROPEAN ELDERBERRY (UNII: BQY1UBX046) (EUROPEAN ELDERBERRY - UNII:BQY1UBX046) EUROPEAN ELDERBERRY 2 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ORANGE JUICE (UNII: 5A9KE2L9L3) 0.6 mL in 30 mL POTASSIUM SORBATE (UNII: 1VPU26JZZ4) 0.03 mL in 30 mL WATER (UNII: 059QF0KO0R) 15.9 mL in 30 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 0.03 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-419-23 1 in 1 BOX 10/08/2011 1 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/27/2010 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-419)