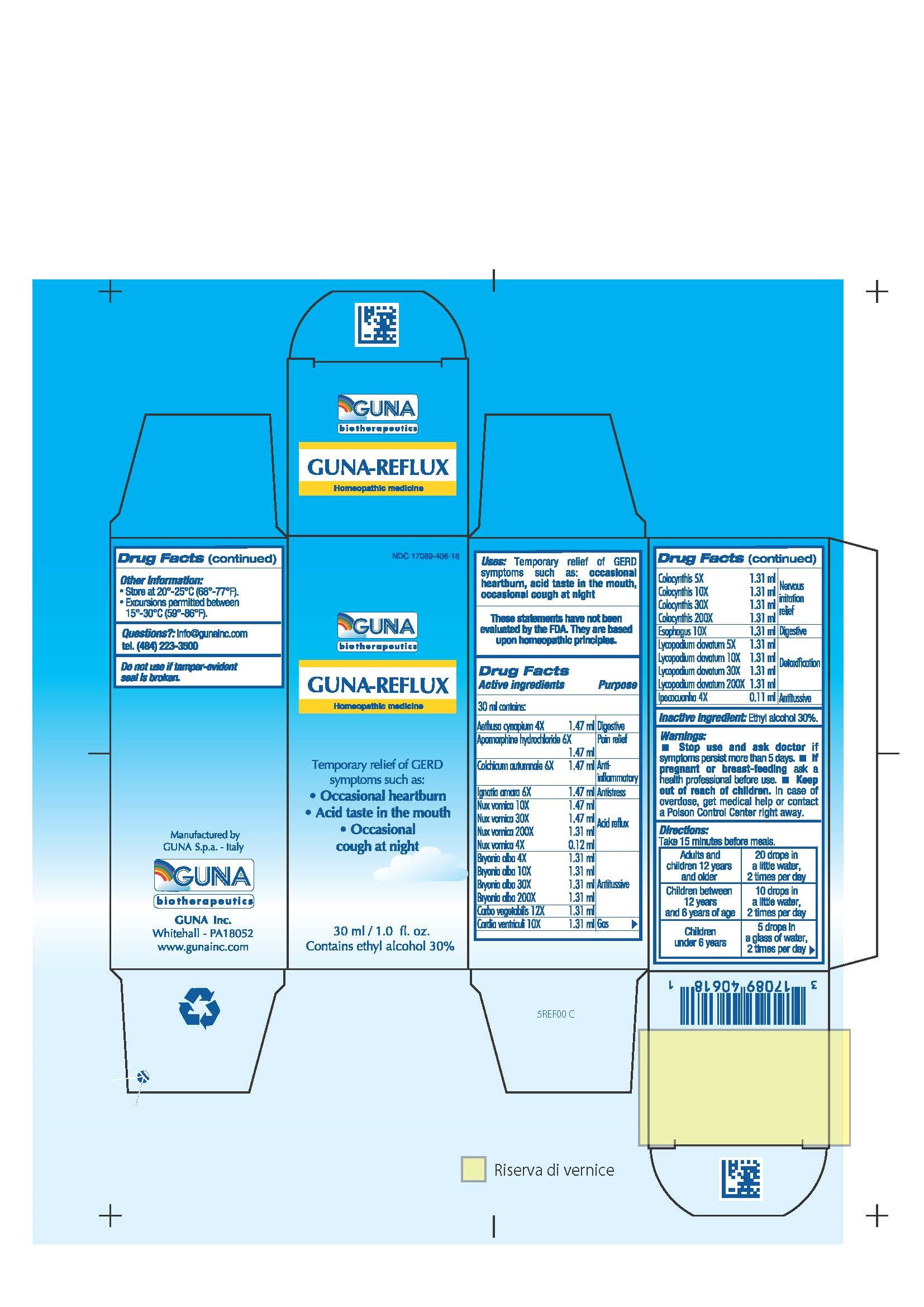
| NDC | 17089-406-18 |
| Set ID | 444c1f4c-dd0c-4525-bb65-c0c211c9487f |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Guna spa |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENTS/PURPOSE
AETHUSA CYNAPIUM 4X DIGESTIVE
APOMORPHINE HYDROCHLORIDE 6X PAIN RELIEF
BRYONIA ALBA 4X, 10X, 30X, 200X ANTITUSSIVE
CARBO VEGETALIS 12X ANTITUSSIVE
CARDIA VENTRICULI 10X GAS
COLCHICUM AUTUMNALE 6X ANTI-INFLAMMATORY
COLOCYNTHIS 5X, 10X, 30X, 200X NERVOUS IRRITATION RELIEF
ESOPHAGUS 10X DIGESTIVE
IGNATIA AMARA 6X ANTISTRESS
IPECACUANHA 4X ANTITUSSIVE
LYCOPODIUM CLAVATUM 5X, 10X, 30X, 200X DETOXIFICATION
NUX VOMICA 4X, 10X, 30X, 200X ACID REFLUX - USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-REFLUX
activated charcoal - aethusa cynapium - apomorphine hydrochloride - bryonia alba root - colchicum autumnale bulb - ipecac - lycopodium clavatum spore - strychnos ignatii seed - strychnos nux-vomica seed - watermelon - sus scrofa stomach - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-406 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AETHUSA CYNAPIUM (UNII: M6936L953C) (AETHUSA CYNAPIUM - UNII:M6936L953C) AETHUSA CYNAPIUM 4 [hp_X] in 30 mL APOMORPHINE HYDROCHLORIDE (UNII: F39049Y068) (APOMORPHINE - UNII:N21FAR7B4S) APOMORPHINE HYDROCHLORIDE 6 [hp_X] in 30 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 30 [hp_X] in 30 mL ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 12 [hp_X] in 30 mL SUS SCROFA STOMACH (UNII: T0920P9Z9A) (SUS SCROFA STOMACH - UNII:T0920P9Z9A) SUS SCROFA STOMACH 10 [hp_X] in 30 mL COLCHICUM AUTUMNALE BULB (UNII: 993QHL78E6) (COLCHICUM AUTUMNALE BULB - UNII:993QHL78E6) COLCHICUM AUTUMNALE BULB 6 [hp_X] in 30 mL WATERMELON (UNII: 231473QB6R) (WATERMELON - UNII:231473QB6R) WATERMELON 30 [hp_X] in 30 mL SUS SCROFA ESOPHAGUS (UNII: 81FZ7X4MWD) (SUS SCROFA ESOPHAGUS - UNII:81FZ7X4MWD) SUS SCROFA ESOPHAGUS 10 [hp_X] in 30 mL STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 6 [hp_X] in 30 mL IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 4 [hp_X] in 30 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 5 [hp_X] in 30 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 4 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 9 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-406-18 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/12/2010 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-406)