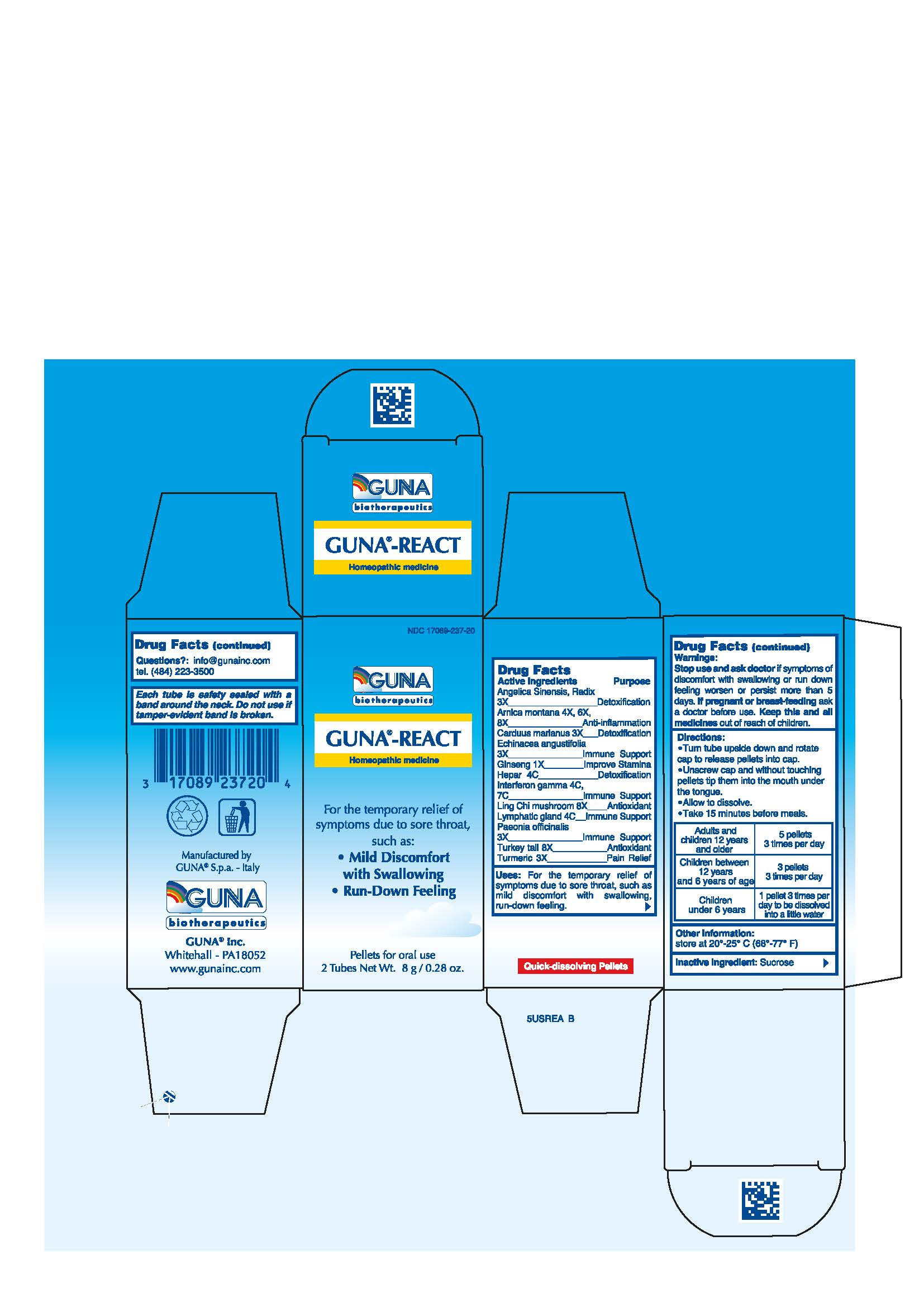
| NDC | 17089-237-20 |
| Set ID | c2b64907-1ae2-4686-b13c-847dbb84724e |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Guna spa |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENTS/PURPOSE
ANGELICA SINENSIS, RADIX 3X DETOXIFICATION
ARNICA MONTANA 4X, 6X, 8X ANTI-INFLAMMATION
CARDUUS MARIANUS 3X DETOXIFICATION
ECHINACEA ANGUSTIFOLIA 3X IMMUNE SUPPORT
GINSENG 1X IMPROVE STAMINA
HEPAR 4C DETOXIFICATION
INTERFERON GAMMA 4C 7C IMMUNE SUPPORT
LING CHI MUSHROOM 8X ANTIOXIDANT
LYMPHATIC GLAND 4C IMMUNE SUPPORT
PAEONIA OFFICINALIS 3X IMMUNE SUPPORT
TURKEY TAIL 8X ANTIOXIDANT
TURMERIC 3X PAIN RELIEF - USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-REACT
angelica sinensis root - arnica montana - asian ginseng - echinacea angustifolia - fomitopsis pinicola fruiting body - interferon gamma-1b - paeonia officinalis root - pork liver - reishi - silybum marianum seed - sus scrofa small intestine mucosa lymph follicle - turmeric - pelletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-237 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TURMERIC (UNII: 856YO1Z64F) (TURMERIC - UNII:856YO1Z64F) TURMERIC 3 [hp_X] in 4 g ANGELICA SINENSIS ROOT (UNII: B66F4574UG) (ANGELICA SINENSIS ROOT - UNII:B66F4574UG) ANGELICA SINENSIS ROOT 3 [hp_X] in 4 g ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 4 [hp_X] in 4 g SILYBUM MARIANUM SEED (UNII: U946SH95EE) (SILYBUM MARIANUM SEED - UNII:U946SH95EE) SILYBUM MARIANUM SEED 3 [hp_X] in 4 g ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 3 [hp_X] in 4 g ASIAN GINSENG (UNII: CUQ3A77YXI) (ASIAN GINSENG - UNII:CUQ3A77YXI) ASIAN GINSENG 1 [hp_X] in 4 g PORK LIVER (UNII: 6EC706HI7F) (PORK LIVER - UNII:6EC706HI7F) PORK LIVER 4 [hp_C] in 4 g INTERFERON GAMMA-1B (UNII: 21K6M2I7AG) (INTERFERON GAMMA-1B - UNII:21K6M2I7AG) INTERFERON GAMMA-1B 4 [hp_C] in 4 g REISHI (UNII: TKD8LH0X2Z) (REISHI - UNII:TKD8LH0X2Z) REISHI 8 [hp_X] in 4 g SUS SCROFA SMALL INTESTINE MUCOSA LYMPH FOLLICLE (UNII: 308LM01C72) (SUS SCROFA SMALL INTESTINE MUCOSA LYMPH FOLLICLE - UNII:308LM01C72) SUS SCROFA SMALL INTESTINE MUCOSA LYMPH FOLLICLE 4 [hp_C] in 4 g PAEONIA OFFICINALIS ROOT (UNII: 8R564U2E1P) (PAEONIA OFFICINALIS ROOT - UNII:8R564U2E1P) PAEONIA OFFICINALIS ROOT 3 [hp_X] in 4 g FOMITOPSIS PINICOLA FRUITING BODY (UNII: 30D02U2IRN) (FOMITOPSIS PINICOLA FRUITING BODY - UNII:30D02U2IRN) FOMITOPSIS PINICOLA FRUITING BODY 8 [hp_X] in 4 g Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) 3.5 g in 4 g Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-237-20 2 in 1 BOX 12/21/2018 1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/23/2006 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-237)