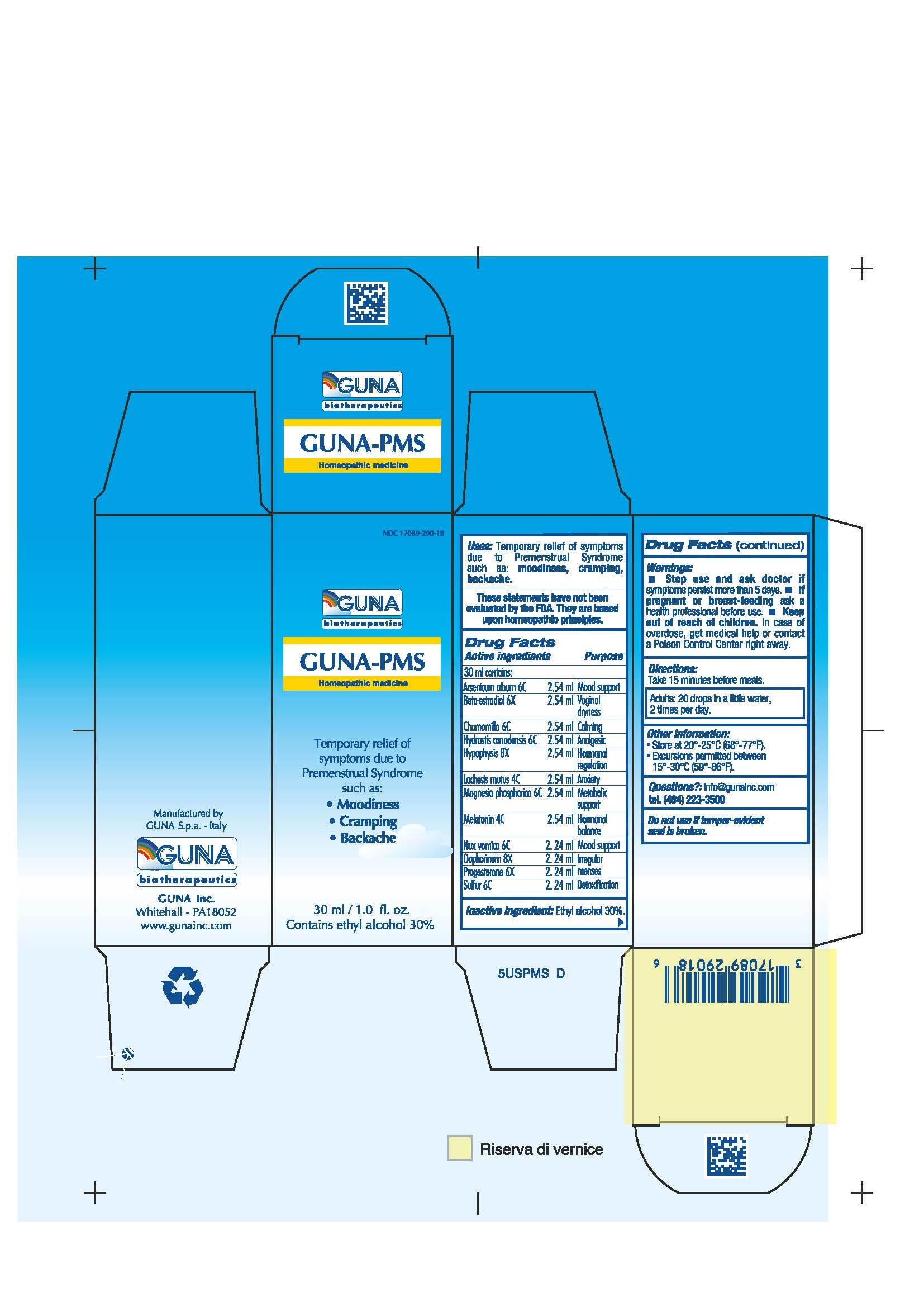
| NDC | 17089-290-18 |
| Set ID | 86f341b1-a8f7-4833-a1be-e51610b05bcd |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Guna spa |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENTS/PURPOSE
ARSENICUM ALBUM 6C MOOD SUPPORT
BETA-ESTRADIOL 6X VAGINAL DRYNESS
CHAMOMILLA 6C CALMING
HYDRASTIS CANADENSIS 6C ANALGESIC
HYPOPHYSIS 8X HORMONAL REGULATION
LACHESIS MUTUS 4C ANXIETY
MAGNESIA PHOSPHORICA 6C METABOLIC SUPPORT
MELATONIN 4C HORMONAL BALANCE
NUX VOMICA 6C MOOD SUPPORT
OOPHORINUM 8X IRREGULAR MENSES
PROGESTERONE 6X IRREGULAR MENSES
SULFUR 6C DETOXIFICATION - USES
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-PMS
arsenic trioxide - estradiol - goldenseal - lachesis muta venom - magnesium phosphate - matricaria recutita - melatonin - progesterone - strychnos nux-vomica seed - sulfur - sus scrofa ovary - sus scrofa pituitary gland - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-290 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 6 [hp_C] in 30 mL ESTRADIOL (UNII: 4TI98Z838E) (ESTRADIOL - UNII:4TI98Z838E) ESTRADIOL 6 [hp_X] in 30 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 6 [hp_C] in 30 mL LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 4 [hp_C] in 30 mL MAGNESIUM PHOSPHATE, TRIBASIC, PENTAHYDRATE (UNII: 453COF7817) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM PHOSPHATE, TRIBASIC, PENTAHYDRATE 6 [hp_C] in 30 mL MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 6 [hp_C] in 30 mL MELATONIN (UNII: JL5DK93RCL) (MELATONIN - UNII:JL5DK93RCL) MELATONIN 4 [hp_C] in 30 mL PROGESTERONE (UNII: 4G7DS2Q64Y) (PROGESTERONE - UNII:4G7DS2Q64Y) PROGESTERONE 6 [hp_X] in 30 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 6 [hp_C] in 30 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 6 [hp_C] in 30 mL SUS SCROFA OVARY (UNII: S7YTV04R8O) (SUS SCROFA OVARY - UNII:S7YTV04R8O) SUS SCROFA OVARY 8 [hp_X] in 30 mL SUS SCROFA PITUITARY GLAND (UNII: E8S87O660T) (SUS SCROFA PITUITARY GLAND - UNII:E8S87O660T) SUS SCROFA PITUITARY GLAND 8 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 9 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-290-18 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/23/2006 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-290)