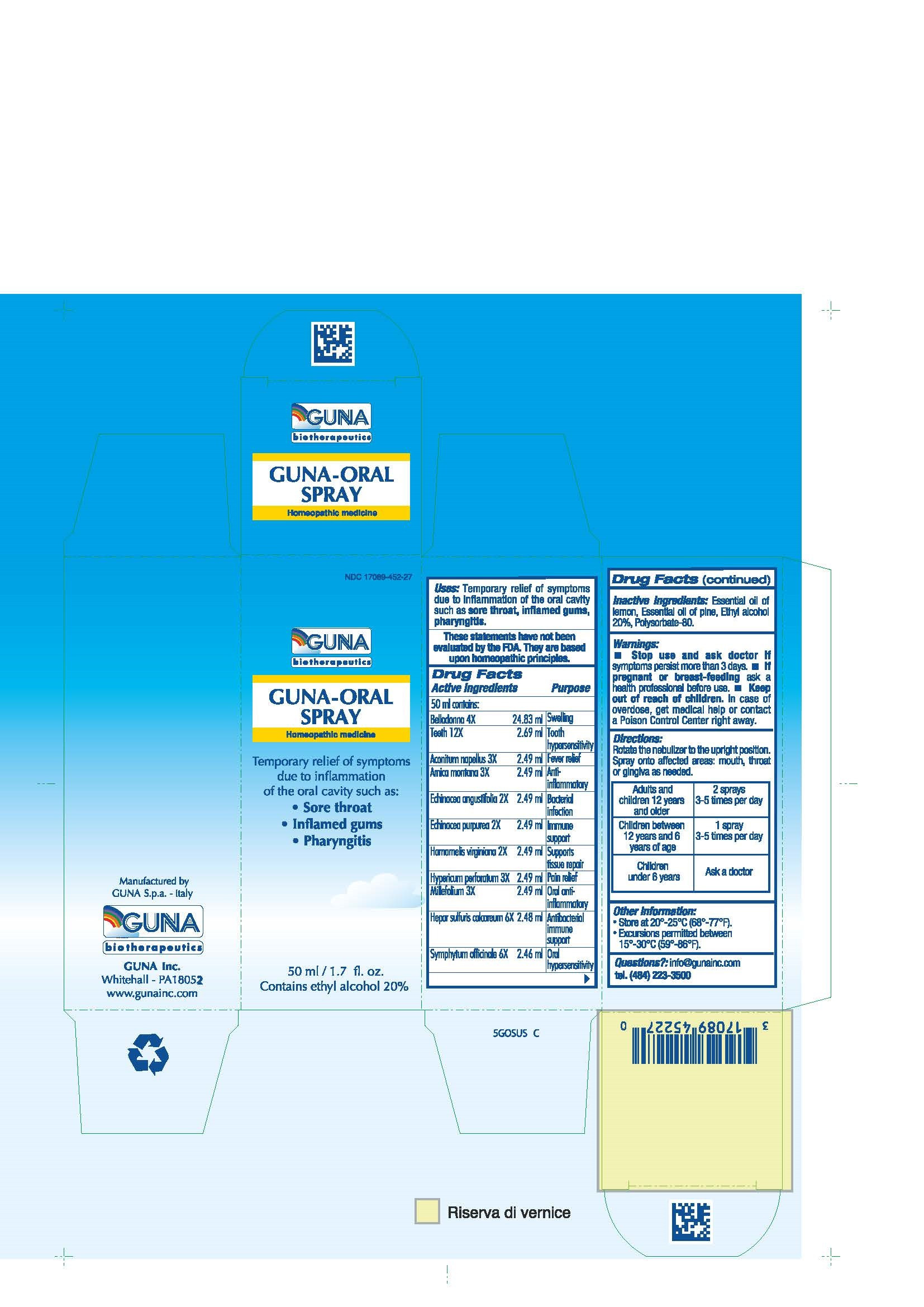
| NDC | 17089-452-27 |
| Set ID | f1b8e5ab-8689-4bf2-a6d9-863d02f29579 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Guna spa |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENTS/PURPOSE
ACONITUM NAPELLUS 3X ANTI-INFLAMMATORY
ARNICA MONTANA 3X FEVER RELIEF
BELLADONNA 4X SWELLING
ECHINACEA ANGUSTIFOLIA 2X BACTERIAL INFECTION
ECHINACEA PURPUREA 2X IMMUNE SUPPORT
HAMAMELIS VIRGINIANA 2X SUPPORTS TISSUE REPAIR
HEPAR SULFURIS CALCAREUM 6X ANTIBACTERIAL IMMUNE SUPPORT
HYPERICUM PERFORATUM 3X PAIN RELIEF
MILLEFOLIUM 3X ORAL ANTI- INFLAMMATORY
SYMPHYTUM OFFICINALE 6X ORAL HYPERSENSITIVITY
TEETH 12X TOOTH HYPERSENSITIVITY - USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-ORAL
achillea millefolium - aconitum napellus - arnica montana - atropa belladonna - calcium sulfide - comfrey root - echinacea angustifolia - echinacea purpurea - hypericum perforatum - sus scrofa tooth - witch hazel - sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-452 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 3 [hp_X] in 30 mL ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 3 [hp_X] in 30 mL ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 4 [hp_X] in 30 mL ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 2 [hp_X] in 30 mL ECHINACEA PURPUREA (UNII: QI7G114Y98) (ECHINACEA PURPUREA - UNII:QI7G114Y98) ECHINACEA PURPUREA 2 [hp_X] in 30 mL WITCH HAZEL (UNII: 101I4J0U34) (WITCH HAZEL - UNII:101I4J0U34) WITCH HAZEL 2 [hp_X] in 30 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 6 [hp_X] in 30 mL HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 3 [hp_X] in 30 mL ACHILLEA MILLEFOLIUM (UNII: 2FXJ6SW4PK) (ACHILLEA MILLEFOLIUM - UNII:2FXJ6SW4PK) ACHILLEA MILLEFOLIUM 3 [hp_X] in 30 mL COMFREY ROOT (UNII: M9VVZ08EKQ) (COMFREY ROOT - UNII:M9VVZ08EKQ) COMFREY ROOT 6 [hp_X] in 30 mL SUS SCROFA TOOTH (UNII: V69U5FL51F) (SUS SCROFA TOOTH - UNII:V69U5FL51F) SUS SCROFA TOOTH 12 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ABIES SACHALINENSIS VAR. SACHALINENSIS OIL (UNII: 9H7TY1ZV7Q) ALCOHOL (UNII: 3K9958V90M) LEMON OIL (UNII: I9GRO824LL) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-452-27 1 in 1 BOX 12/21/2018 1 50 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/27/2010 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-452)