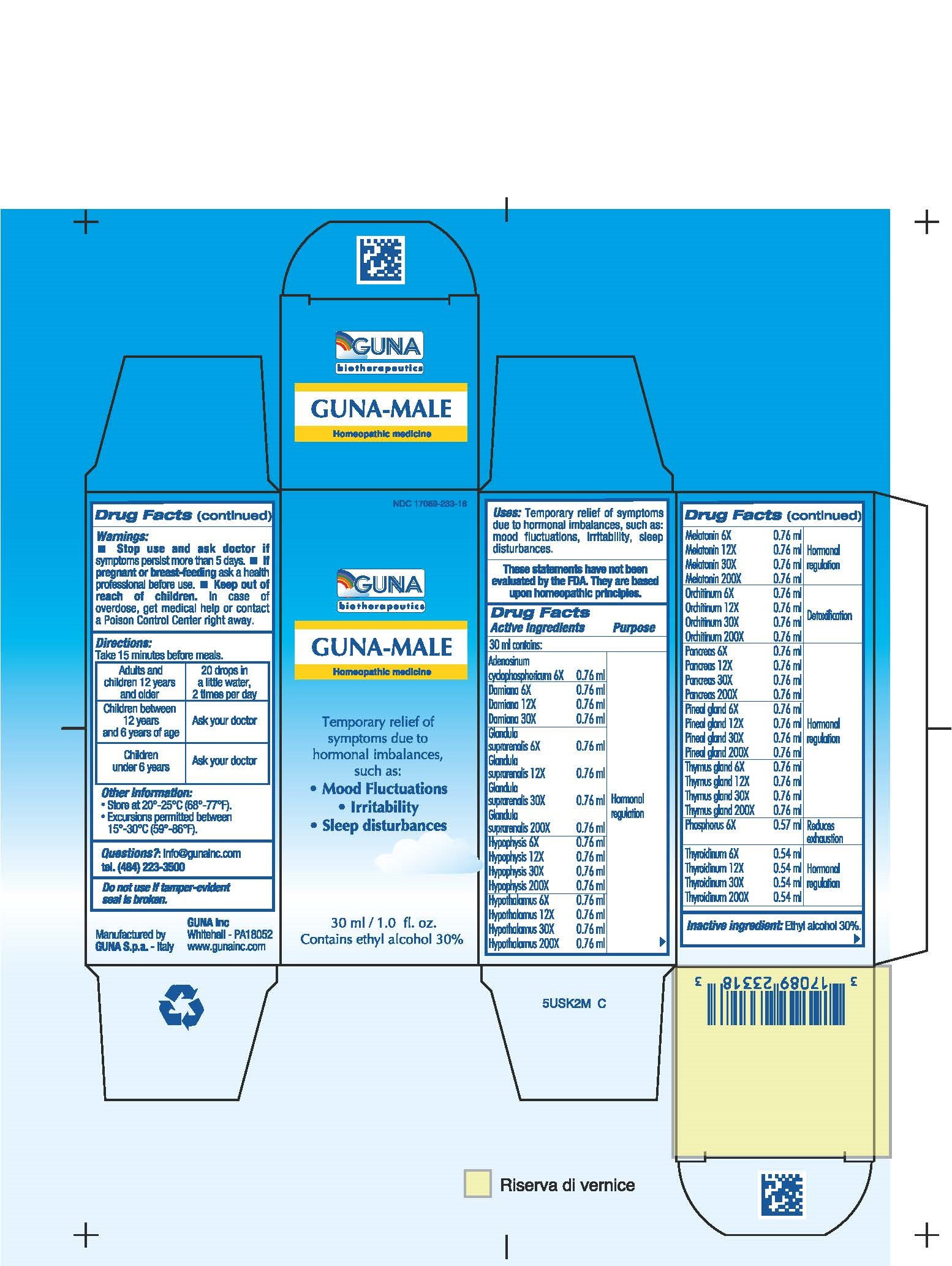
| NDC | 17089-233-18 |
| Set ID | 176425c5-0be9-4cc2-be71-599d4d135f23 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Guna spa |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENTS/PURPOSE
ADENOSINUM CYCLOPHOSPHORICUM 6X HORMONAL REGULATION
DAMIANA 6X 12X 30X HORMONAL REGULATION
GLANDULA SUPRARENALIS 6X 12X 30X 200X HORMONAL REGULATION
HYPOPHYSIS 6X 12X 30X 200X HORMONAL REGULATION
HYPOTHALAMUS 6X 12X 30X 200X HORMONAL REGULATION
MELATONIN 6X 12X 30X 200X HORMONAL REGULATION
ORCHITINUM 6X 12X 30X 200X DETOXIFICATION
PANCREAS 6X, 12X, 30X, 200X HORMONAL REGULATION
PHOSPHORUS 6X REDUCES EXHAUSTION
PINEAL GLAND 6X 12X 30X 200X HORMONAL REGULATION
THYMUS GLAND 6X 12X 30X 200X HORMONAL REGULATION
THYROIDINUM 6X 12X 30X 200X HORMONAL REGULATION - USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-MALE
adenosinum ciclophosphoricum - melatonin - phosphorus - sus scrofa adrenal gland - sus scrofa hypothalamus - sus scrofa pancreas - sus scrofa pineal gland - sus scrofa pituitary gland - sus scrofa testicle - sus scrofa thymus - thyroid - turnera diffusa top - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-233 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ADENOSINE CYCLIC PHOSPHATE (UNII: E0399OZS9N) (ADENOSINE CYCLIC PHOSPHATE - UNII:E0399OZS9N) ADENOSINE CYCLIC PHOSPHATE 6 [hp_X] in 30 mL TURNERA DIFFUSA LEAFY TWIG (UNII: RQ2CFA7WWJ) (TURNERA DIFFUSA LEAFY TWIG - UNII:RQ2CFA7WWJ) TURNERA DIFFUSA LEAFY TWIG 6 [hp_X] in 30 mL SUS SCROFA ADRENAL GLAND (UNII: 398IYQ16YV) (SUS SCROFA ADRENAL GLAND - UNII:398IYQ16YV) SUS SCROFA ADRENAL GLAND 30 [hp_X] in 30 mL SUS SCROFA PITUITARY GLAND (UNII: E8S87O660T) (SUS SCROFA PITUITARY GLAND - UNII:E8S87O660T) SUS SCROFA PITUITARY GLAND 30 [hp_X] in 30 mL SUS SCROFA HYPOTHALAMUS (UNII: N6R0856Z79) (SUS SCROFA HYPOTHALAMUS - UNII:N6R0856Z79) SUS SCROFA HYPOTHALAMUS 30 [hp_X] in 30 mL MELATONIN (UNII: JL5DK93RCL) (MELATONIN - UNII:JL5DK93RCL) MELATONIN 30 [hp_X] in 30 mL SUS SCROFA TESTICLE (UNII: KM02613O28) (SUS SCROFA TESTICLE - UNII:KM02613O28) SUS SCROFA TESTICLE 30 [hp_X] in 30 mL SUS SCROFA PANCREAS (UNII: 9Y3J3362RY) (SUS SCROFA PANCREAS - UNII:9Y3J3362RY) SUS SCROFA PANCREAS 30 [hp_X] in 30 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 6 [hp_X] in 30 mL SUS SCROFA PINEAL GLAND (UNII: 050QZ2EDK7) (SUS SCROFA PINEAL GLAND - UNII:050QZ2EDK7) SUS SCROFA PINEAL GLAND 30 [hp_X] in 30 mL SUS SCROFA THYMUS (UNII: 7B69B0BD62) (SUS SCROFA THYMUS - UNII:7B69B0BD62) SUS SCROFA THYMUS 30 [hp_X] in 30 mL THYROID (UNII: 0B4FDL9I6P) (THYROID - UNII:0B4FDL9I6P) THYROID 30 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 9 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-233-18 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/23/2006 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-233)