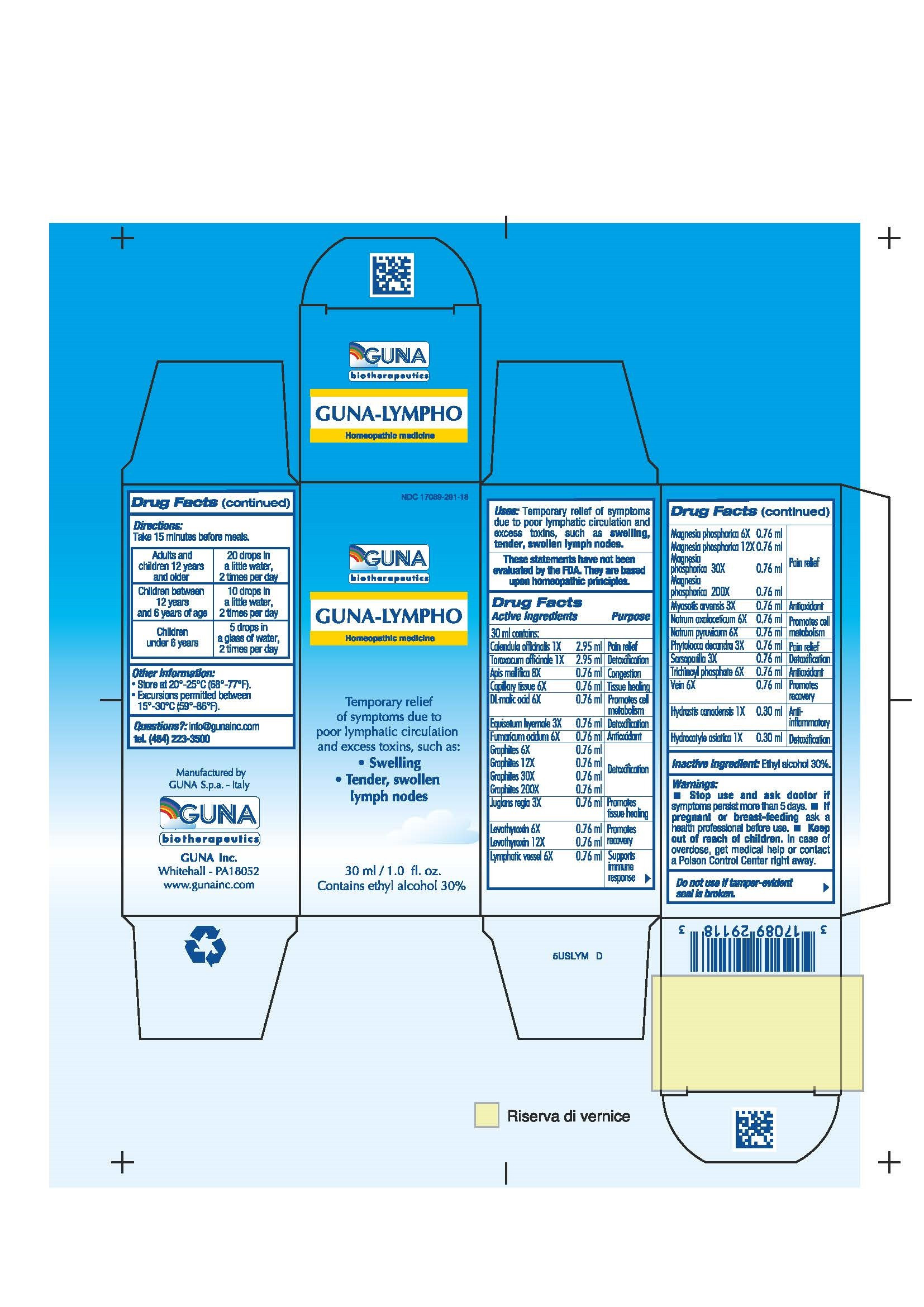
| NDC | 17089-291-18 |
| Set ID | ecd11761-9b9c-4d42-9e2b-f03ba4fed8ae |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Guna spa |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENTS/PURPOSE
APIS MELLIFICA 8X CONGESTION
CALENDULA OFFICINALIS 1X PAIN RELIEF
CAPILLARY TISSUE 6X TISSUE HEALING
DL-MALIC ACID 6X PROMOTES CELL METABOLISM
EQUISETUM HYEMALE 3X DETOXIFICATION
FUMARICUM ACIDUM 6X ANTIOXIDANT
GRAPHITES 6X 12X 30X 200X DETOXIFICATION
HYDRASTIS CANADENSIS 1X ANTI-INFLAMMATORY
HYDROCOTYLE ASIATICA 1X DETOXIFICATION
JUGLANS REGIA 3X PROMOTES TISSUE HEALING
LEVOTHYROXIN 6X 12X PROMOTES RECOVERY
LYMPHATIC VESSEL 6X SUPPORTS IMMUNE RESPONSE
MAGNESIA PHOSPHORICA 6X 12X 30X 200X PAIN RELIEF
MYOSOTIS ARVENSIS 3X ANTIOXIDANT
NATRUM OXALACETICUM 6X PROMOTES CELL METABOLISM
NATRUM PYRUVICUM 6X PROMOTES CELL METABOLISM
PHYTOLACCA DECANDRA 3X PAIN RELIEF
SARSAPARILLA 3X DETOXIFICATION
TARAXACUM OFFICINALE 1X DETOXIFICATION
TRICHINOYL PHOSPHATE 6X ANTIOXIDANT
VEIN 6X PROMOTES RECOVERY - USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-LYMPHO
apis mellifera - calendula officinalis flower - centella asiatica - dodecahydroxycyclohexane dihydrate - equisetum hyemale - fumaric acid - goldenseal - graphite - juglans regia leaf - levothyroxine - magnesium phosphate - malic acid - myosotis arvensis - phytolacca americana root - sarsaparilla - sodium diethyl oxalacetate - sodium pyruvate - sus scrofa capillary tissue - sus scrofa small intestine mucosa lymph follicle - sus scrofa vein - taraxacum officinale - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-291 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 8 [hp_X] in 30 mL CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) (CALENDULA OFFICINALIS FLOWER - UNII:P0M7O4Y7YD) CALENDULA OFFICINALIS FLOWER 1 [hp_X] in 30 mL SUS SCROFA CAPILLARY TISSUE (UNII: 253A0356PN) (SUS SCROFA CAPILLARY TISSUE - UNII:253A0356PN) SUS SCROFA CAPILLARY TISSUE 6 [hp_X] in 30 mL MALIC ACID (UNII: 817L1N4CKP) (MALIC ACID - UNII:817L1N4CKP) MALIC ACID 6 [hp_X] in 30 mL EQUISETUM HYEMALE (UNII: 59677RXH25) (EQUISETUM HYEMALE - UNII:59677RXH25) EQUISETUM HYEMALE 3 [hp_X] in 30 mL FUMARIC ACID (UNII: 88XHZ13131) (FUMARIC ACID - UNII:88XHZ13131) FUMARIC ACID 6 [hp_X] in 30 mL GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 30 [hp_X] in 30 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 1 [hp_X] in 30 mL CENTELLA ASIATICA (UNII: 7M867G6T1U) (CENTELLA ASIATICA - UNII:7M867G6T1U) CENTELLA ASIATICA 1 [hp_X] in 30 mL JUGLANS REGIA LEAF (UNII: 85HKB87105) (JUGLANS REGIA LEAF - UNII:85HKB87105) JUGLANS REGIA LEAF 3 [hp_X] in 30 mL LEVOTHYROXINE (UNII: Q51BO43MG4) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE 12 [hp_X] in 30 mL SUS SCROFA SMALL INTESTINE MUCOSA LYMPH FOLLICLE (UNII: 308LM01C72) (SUS SCROFA SMALL INTESTINE MUCOSA LYMPH FOLLICLE - UNII:308LM01C72) SUS SCROFA SMALL INTESTINE MUCOSA LYMPH FOLLICLE 6 [hp_X] in 30 mL MAGNESIUM PHOSPHATE, TRIBASIC, PENTAHYDRATE (UNII: 453COF7817) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM PHOSPHATE, TRIBASIC, PENTAHYDRATE 6 [hp_X] in 30 mL MYOSOTIS ARVENSIS (UNII: C73BK97H5J) (MYOSOTIS ARVENSIS - UNII:C73BK97H5J) MYOSOTIS ARVENSIS 3 [hp_X] in 30 mL SODIUM DIETHYL OXALACETATE (UNII: 6CA025Y4FG) (DIETHYL OXALACETATE - UNII:15S56468G7) SODIUM DIETHYL OXALACETATE 6 [hp_X] in 30 mL SODIUM PYRUVATE (UNII: POD38AIF08) (PYRUVIC ACID - UNII:8558G7RUTR) SODIUM PYRUVATE 6 [hp_X] in 30 mL PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 3 [hp_X] in 30 mL SARSAPARILLA (UNII: 2H1576D5WG) (SARSAPARILLA - UNII:2H1576D5WG) SARSAPARILLA 3 [hp_X] in 30 mL TARAXACUM OFFICINALE (UNII: 39981FM375) (TARAXACUM OFFICINALE - UNII:39981FM375) TARAXACUM OFFICINALE 1 [hp_X] in 30 mL DODECAHYDROXYCYCLOHEXANE DIHYDRATE (UNII: 5BWD2J7B4W) (DODECAHYDROXYCYCLOHEXANE - UNII:I1Z9VS3H64) DODECAHYDROXYCYCLOHEXANE DIHYDRATE 6 [hp_X] in 30 mL SUS SCROFA VEIN (UNII: 2510RH3I89) (SUS SCROFA VEIN - UNII:2510RH3I89) SUS SCROFA VEIN 6 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 9 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-291-18 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/23/2006 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-291)