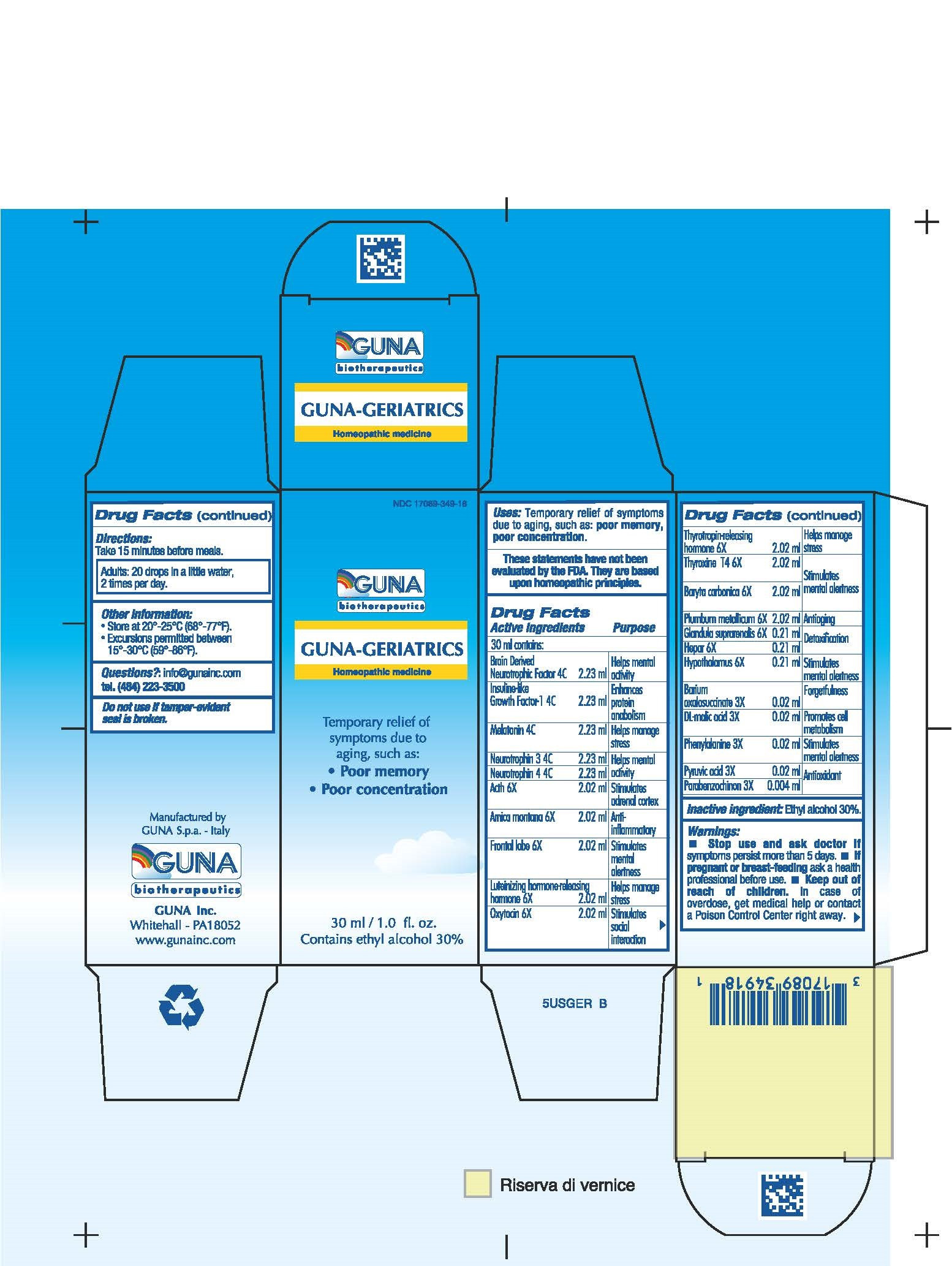
| NDC | 17089-349-18 |
| Set ID | 03dc86d3-575f-4c65-b06d-2613efbcd943 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Guna spa |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENTS/PURPOSE
ACTH 6X ADRENAL SUPPORT
ARNICA MONTANA 6X ANTI-INFLAMMATORY
BARIUM OXALOSUCCINATE 3X FORGETFULNESS
BARYTA CARBONICA 6X IMPROVES MENTAL ATTENTION
BRAIN DERIVED NEUROTROPHIC FACTOR 4C HELPS MENTAL ACTIVITY
DL-MALIC ACID 3X PROMOTE CELL METABOLISM
FRONTAL LOBE 6X STIMULATES MENTAL ALERTNESS
GLANDULA SUPRARENALIS 6X DETOXIFICATION
HEPAR 6X DETOXIFICATION
HYPOTHALAMUS 6X IMPROVES MENTAL ATTENTION
INSULINE-LIKE GROWTH FACTOR-1 4C ENHANCES PROTEIN ANABOLISM
LUTEINIZING HORMONE-RELEASING HORMONE 6X HELPS MANAGE STRESS
MELATONIN 4C HELPS MANAGE STRESS
NEUROTROPHIN 3 4C HELPS MENTAL ACTIVITY
NEUROTROPHIN 4 4C HELPS MENTAL ACTIVITY
OXYTOCIN 6X STIMULATES SOCIAL RECOGNITION
PARABENZOCHINON 3X ANTIOXIDANT
PHENYLALANINE 3X STIMULATES MENTAL ALERTNESS
PLUMBUM METALLICUM 6X ANTIAGING
PYRUVIC ACID 3X ANTIOXIDANT
THYROTROPIN-RELEASING HORMONE 6X HELPS MANAGE STRESS
THYROXINE T4 6X STIMULATES MENTAL ALERTNESS - USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INACTIVE INGREDIENT
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-GERIATRICS
1,4-benzoquinone - arnica montana - oxytocin - barium carbonate - barium oxalosuccinate - corticotropin - lead - levothyroxine - lutrelin - malic acid - melatonin - neurotrophin-3 - neurotrophin-4 - phenylalanine - pork liver - pyruvic acid - rinfabate - sus scrofa adrenal gland - sus scrofa frontal lobe - sus scrofa hypothalamus - thyrotropin alfa - brain-derived neurotrophic factor human - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-349 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength QUINONE (UNII: 3T006GV98U) (QUINONE - UNII:3T006GV98U) QUINONE 3 [hp_X] in 30 mL BARIUM OXALOSUCCINATE (UNII: L7A49804ZQ) (BARIUM CATION - UNII:V645272HLN) BARIUM CATION 3 [hp_X] in 30 mL CORTICOTROPIN (UNII: K0U68Q2TXA) (CORTICOTROPIN - UNII:K0U68Q2TXA) CORTICOTROPIN 6 [hp_X] in 30 mL ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 6 [hp_X] in 30 mL BARIUM CARBONATE (UNII: 6P669D8HQ8) (BARIUM CATION - UNII:V645272HLN) BARIUM CARBONATE 6 [hp_X] in 30 mL BRAIN-DERIVED NEUROTROPHIC FACTOR HUMAN (UNII: 7171WSG8A2) (BRAIN-DERIVED NEUROTROPHIC FACTOR HUMAN - UNII:7171WSG8A2) BRAIN-DERIVED NEUROTROPHIC FACTOR HUMAN 4 [hp_C] in 30 mL MALIC ACID (UNII: 817L1N4CKP) (MALIC ACID - UNII:817L1N4CKP) MALIC ACID 3 [hp_X] in 30 mL SUS SCROFA FRONTAL LOBE (UNII: GV54Q19G55) (SUS SCROFA FRONTAL LOBE - UNII:GV54Q19G55) SUS SCROFA FRONTAL LOBE 6 [hp_X] in 30 mL SUS SCROFA ADRENAL GLAND (UNII: 398IYQ16YV) (SUS SCROFA ADRENAL GLAND - UNII:398IYQ16YV) SUS SCROFA ADRENAL GLAND 6 [hp_X] in 30 mL PORK LIVER (UNII: 6EC706HI7F) (PORK LIVER - UNII:6EC706HI7F) PORK LIVER 6 [hp_X] in 30 mL SUS SCROFA HYPOTHALAMUS (UNII: N6R0856Z79) (SUS SCROFA HYPOTHALAMUS - UNII:N6R0856Z79) SUS SCROFA HYPOTHALAMUS 6 [hp_X] in 30 mL RINFABATE (UNII: KU1MBT4JJB) (RINFABATE - UNII:KU1MBT4JJB) RINFABATE 4 [hp_C] in 30 mL LUTRELIN (UNII: QH51543Y7U) (LUTRELIN - UNII:QH51543Y7U) LUTRELIN 6 [hp_X] in 30 mL MELATONIN (UNII: JL5DK93RCL) (MELATONIN - UNII:JL5DK93RCL) MELATONIN 4 [hp_C] in 30 mL NEUROTROPHIN-3 (UNII: 02R4V6T25Y) (NEUROTROPHIN-3 - UNII:02R4V6T25Y) NEUROTROPHIN-3 4 [hp_C] in 30 mL NEUROTROPHIN-4 (UNII: P658DCA9XD) (NEUROTROPHIN-4 - UNII:P658DCA9XD) NEUROTROPHIN-4 4 [hp_C] in 30 mL OXYTOCIN (UNII: 1JQS135EYN) (OXYTOCIN - UNII:1JQS135EYN) OXYTOCIN 6 [hp_X] in 30 mL PHENYLALANINE (UNII: 47E5O17Y3R) (PHENYLALANINE - UNII:47E5O17Y3R) PHENYLALANINE 3 [hp_X] in 30 mL LEAD (UNII: 2P299V784P) (LEAD - UNII:2P299V784P) LEAD 6 [hp_X] in 30 mL PYRUVIC ACID (UNII: 8558G7RUTR) (PYRUVIC ACID - UNII:8558G7RUTR) PYRUVIC ACID 3 [hp_X] in 30 mL THYROTROPIN ALFA (UNII: AVX3D5A4LM) (THYROTROPIN ALFA - UNII:AVX3D5A4LM) THYROTROPIN ALFA 6 [hp_X] in 30 mL LEVOTHYROXINE (UNII: Q51BO43MG4) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE 6 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-349-18 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/16/2008 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-349)