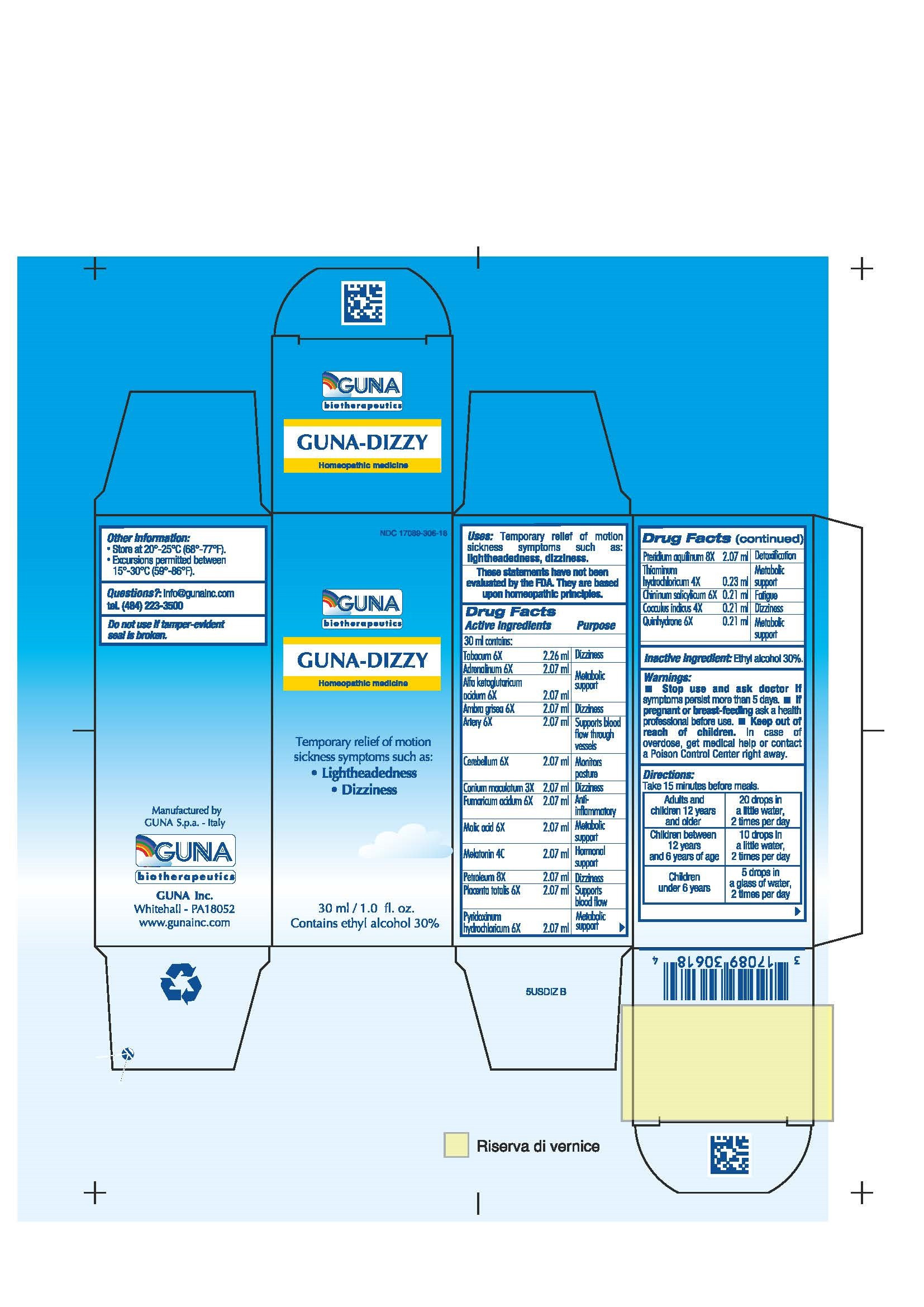
| NDC | 17089-306-18 |
| Set ID | c5f4a798-e628-4388-a98d-7449dd92f2d6 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Guna spa |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENTS/PURPOSE
ADRENALINUM 6X METABOLIC SUPPORT
ALFA KETOGLUTARICUM ACIDUM 6X METABOLIC SUPPORT
AMBRA GIRSEA 6X DIZZINESS
ARTERY 6X SUPPORTS BLOOD FLOW THROUGH VESSELS
CEREBELLUM 6X MONITORS POSTURE
CHININUM SALICYLICUM 6X FATIGUE
COCCULUS INDICUS 4X DIZZINESS
CONIUM MACULATUM 3X DIZZINESS
FUMARICUM ACIDUM 6X ANTI-INFLAMMATORY
MALIC ACID 6X METABOLIC SUPPORT
MELATONIN 4C HORMONAL SUPPORT
PETROLEUM 8X DIZZINESS
PLACENTA TOTALIS 6X SUPPORTS BLOOD FLOW
PTERIDIUM AQUILINUM 8X DETOXIFICATION
PYRIDOXINUM HYDROCHLORICUM 6X METABOLIC SUPPORT
QUINHYDRONE 6X METABOLIC SUPPORT
TABACUM 6X DIZZINESS
THIAMINUM HYDROCHLORICUM 4X METABOLIC SUPPORT - USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-DIZZY
.alpha.-ketoglutaric acid - ambergris - anamirta cocculus seed - conium maculatum flowering top - epinephrine - fumaric acid - malic acid - melatonin - mineral oil - pteridium aquilinum root - pyridoxine hydrochloride - quinhydrone - quinine - sus scrofa artery - sus scrofa cerebellum - sus scrofa placenta - thiamine hydrochloride - tobacco leaf - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-306 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE (UNII: YKH834O4BH) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 6 [hp_X] in 30 mL .ALPHA.-KETOGLUTARIC ACID (UNII: 8ID597Z82X) (.ALPHA.-KETOGLUTARIC ACID - UNII:8ID597Z82X) .ALPHA.-KETOGLUTARIC ACID 6 [hp_X] in 30 mL AMBERGRIS (UNII: XTC0D02P6C) (AMBERGRIS - UNII:XTC0D02P6C) AMBERGRIS 6 [hp_X] in 30 mL SUS SCROFA ARTERY (UNII: 63O327782Q) (SUS SCROFA ARTERY - UNII:63O327782Q) SUS SCROFA ARTERY 6 [hp_X] in 30 mL SUS SCROFA CEREBELLUM (UNII: 49NGK53TPQ) (SUS SCROFA CEREBELLUM - UNII:49NGK53TPQ) SUS SCROFA CEREBELLUM 6 [hp_X] in 30 mL QUININE SALICYLATE (UNII: 6DY04L71DR) (QUININE - UNII:A7V27PHC7A) QUININE SALICYLATE 6 [hp_X] in 30 mL ANAMIRTA COCCULUS SEED (UNII: 810258W28U) (ANAMIRTA COCCULUS SEED - UNII:810258W28U) ANAMIRTA COCCULUS SEED 4 [hp_X] in 30 mL CONIUM MACULATUM FLOWERING TOP (UNII: Q28R5GF371) (CONIUM MACULATUM FLOWERING TOP - UNII:Q28R5GF371) CONIUM MACULATUM FLOWERING TOP 3 [hp_X] in 30 mL FUMARIC ACID (UNII: 88XHZ13131) (FUMARIC ACID - UNII:88XHZ13131) FUMARIC ACID 6 [hp_X] in 30 mL MALIC ACID (UNII: 817L1N4CKP) (MALIC ACID - UNII:817L1N4CKP) MALIC ACID 6 [hp_X] in 30 mL MELATONIN (UNII: JL5DK93RCL) (MELATONIN - UNII:JL5DK93RCL) MELATONIN 4 [hp_C] in 30 mL MINERAL OIL (UNII: T5L8T28FGP) (MINERAL OIL - UNII:T5L8T28FGP) MINERAL OIL 8 [hp_X] in 30 mL SUS SCROFA PLACENTA (UNII: C8CV8867O8) (SUS SCROFA PLACENTA - UNII:C8CV8867O8) SUS SCROFA PLACENTA 6 [hp_X] in 30 mL PTERIDIUM AQUILINUM ROOT (UNII: HQJ5Z3SOIV) (PTERIDIUM AQUILINUM ROOT - UNII:HQJ5Z3SOIV) PTERIDIUM AQUILINUM ROOT 8 [hp_X] in 30 mL PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 6 [hp_X] in 30 mL QUINHYDRONE (UNII: P4A66LQ3QJ) (HYDROQUINONE - UNII:XV74C1N1AE) QUINHYDRONE 6 [hp_X] in 30 mL TOBACCO LEAF (UNII: 6YR2608RSU) (TOBACCO LEAF - UNII:6YR2608RSU) TOBACCO LEAF 6 [hp_X] in 30 mL THIAMINE HYDROCHLORIDE (UNII: M572600E5P) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE HYDROCHLORIDE 4 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 9 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-306-18 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/23/2006 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-306)