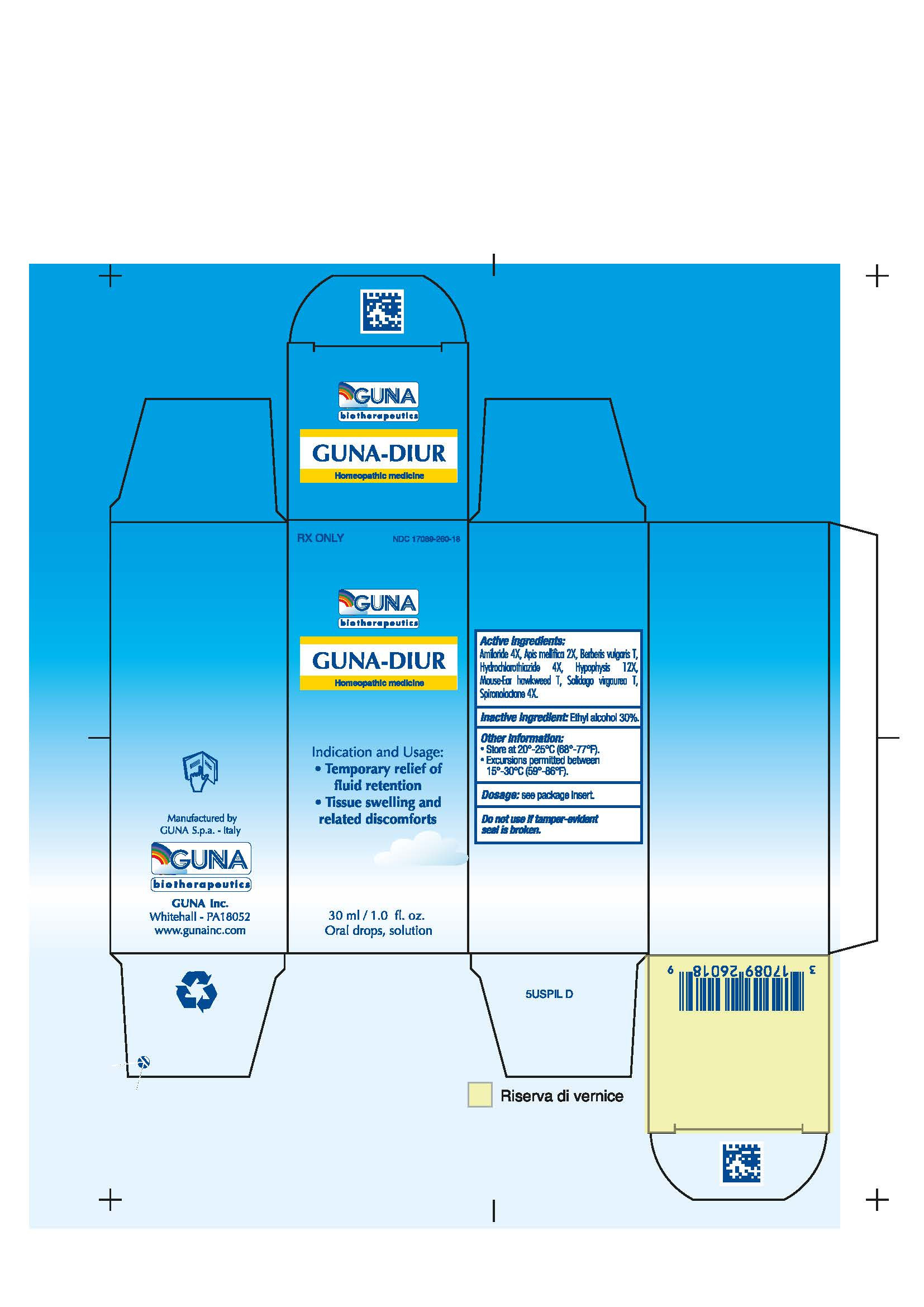
| NDC | 17089-260-18 |
| Set ID | 3f91cd92-eb6d-4bda-9cad-2e11e0b0fbe2 |
| Category | HUMAN PRESCRIPTION DRUG LABEL |
| Packager | Guna spa |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- 1. INDICATIONS AND USAGE
- 2. DOSAGE AND ADMINISTRATION
-
3. DOSAGE FORMS AND STRENGTHS
3.1. 30 ml Bottle dropper container contains:
Active ingredients: Amiloride 4X 0.006 ml, Apis Mellifica 2X 0.626 ml, Berberis Vulgaris T 0.314 ml, Hydrochlorothiazide 4X 0.006 ml, Hypophysis 12X 6.314 ml, Mouse-Ear Hawkweed T 6.314 ml, Solidago Virgaurea T 0.314 ml, Spironolactone 4X 0.006 ml.
Inactive Ingredient: Ethylic Alcohol 30% - 4. CONTRAINDICATIONS
- 5. WARNINGS AND PRECAUTIONS
- 6. ADVERSE REACTIONS
- 7. DRUG INTERACTIONS
-
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy: Pregnancy category C. Animal reproduction studies have not been conducted with GUNA-DIUR. GUNA®- DIUR should not be given to a pregnant woman.
8.2. Lactation: It is not known whether any of the ingredients in GUNA- DIUR are secreted in human milk. However, since many drugs are secreted in human milk, caution should be exercised when GUNA- DIUR is administered to a nursing woman.
8.3. Pediatric use: Safety and effectiveness in pediatric patients have not been established.
8.4. Geriatric use: No restrictions. - 9. DRUG ABUSE AND DEPENDENCE
- 10. OVERDOSAGE
- 11. DESCRIPTION
- 12. CLINICAL PHARMACOLOGY
- 13. NONCLINICAL TOXICOLOGY
- 14. CLINICAL STUDIES
- 15. REFERENCES
- 16. HOW SUPPLIED/STORAGE AND HANDLING
- 17. PATIENT COUNSELING INFORMATION
- PACKAGE LABEL
-
INGREDIENTS AND APPEARANCE
GUNA-DIUR
amiloride - apis mellifera - berberis vulgaris fruit - hieracium pilosella flowering top - hydrochlorothiazide - solidago virgaurea flowering top - spironolactone - sus scrofa pituitary gland - solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:17089-260 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMILORIDE (UNII: 7DZO8EB0Z3) (AMILORIDE - UNII:7DZO8EB0Z3) AMILORIDE 4 [hp_X] in 30 mL APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 2 [hp_X] in 30 mL BERBERIS VULGARIS FRUIT (UNII: 6XEF22AHC3) (BERBERIS VULGARIS FRUIT - UNII:6XEF22AHC3) BERBERIS VULGARIS FRUIT 0.3 g in 30 mL HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 4 [hp_X] in 30 mL SUS SCROFA PITUITARY GLAND (UNII: E8S87O660T) (SUS SCROFA PITUITARY GLAND - UNII:E8S87O660T) SUS SCROFA PITUITARY GLAND 12 [hp_X] in 30 mL HIERACIUM PILOSELLA FLOWERING TOP (UNII: 08A7Y81S1P) (HIERACIUM PILOSELLA FLOWERING TOP - UNII:08A7Y81S1P) HIERACIUM PILOSELLA FLOWERING TOP 0.3 g in 30 mL SOLIDAGO VIRGAUREA FLOWERING TOP (UNII: 5405K23S50) (SOLIDAGO VIRGAUREA FLOWERING TOP - UNII:5405K23S50) SOLIDAGO VIRGAUREA FLOWERING TOP 0.3 g in 30 mL SPIRONOLACTONE (UNII: 27O7W4T232) (SPIRONOLACTONE - UNII:27O7W4T232) SPIRONOLACTONE 4 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-260-18 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/23/2006 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-260)