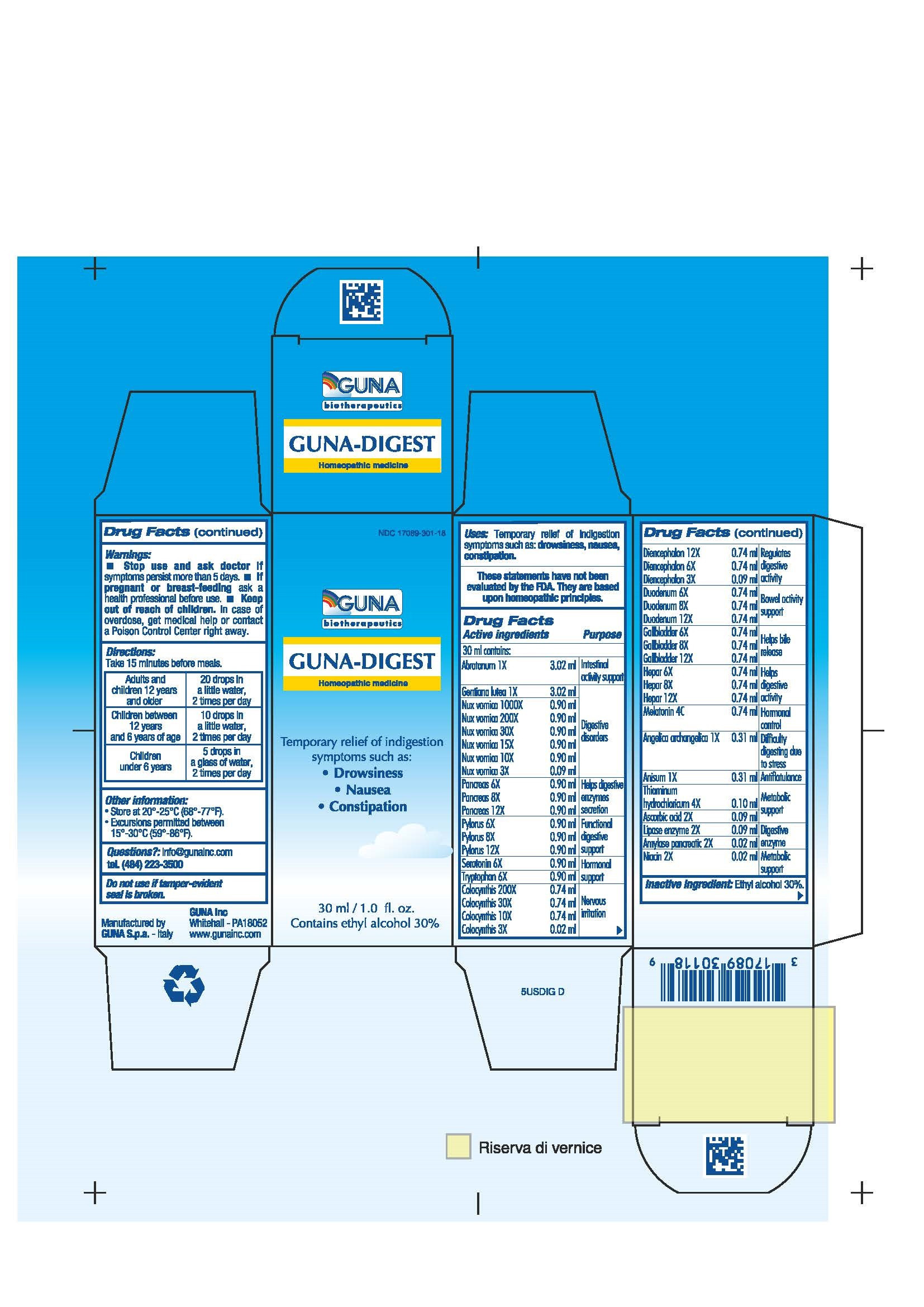
| NDC | 17089-301-18 |
| Set ID | 624228b9-70be-43a1-93a5-f6e708814fa2 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Guna spa |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENTS/PURPOSE
ABROTANUM 1X INTESTINAL ACTIVITY SUPPORT
AMYLASE PANCREATIC 2X DIGESTIVE ENZYME
ANGELICA ARCHANGELICA 1X DIFFICULTY DIGESTING DUE TO STRESS
ANISUM 1X ANTIFLATULANCE
ASCORBIC ACID 2X METABOLIC SUPPORT
COLOCYNTHIS 3X 10X 30X 200X NERVOUS IRRITATION
DIENCEPHALON 3X 6X 12X REGULATES DIGESTIVE ACTIVITY
DUODENUM 6X 8X 12X BOWEL ACTIVITY SUPPORT
GALL BLADDER 6X 8X 12X HELPS BILE RELEASE
GENTIANA LUTEA 1X DIGESTIVE DISORDERS
HEPAR 6X 8X 12X HELPS DIGESTIVE ACTIVITY
LIPASE ENZYME 2X DIGESTIVE ENZYME
MELATONIN 4C HORMONAL CONTROL
NIACIN 2X METABOLIC SUPPORT
NUX VOMICA 3X 10X 15X 30X 200X 1000X DIGESTIVE DISORDERS
PANCREAS 6X 8X 12X HELPS DIGESTIVE ENZYMES SECRETION
PYLORUS 6X 8X 12X FUNCTIONAL DIGESTIVE SUPPORT
SEROTONIN 6X HORMONAL SUPPORT
THIAMINUM HYDROCHLORICUM 4X METABOLIC SUPPORT
TRYPSIN 2X DIGESTIVE ENZYME
TRYPTOPHAN 6X HORMONAL SUPPORT - USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-DIGEST
angelica archangelica root - anisum - artemisia abrotanum flowering top - ascorbic acid - citrullus colocynthis fruit pulp - gentiana lutea root - melatonin - niacin - pancrelipase amylase - pancrelipase lipase - pork liver - serotonin - strychnos nux-vomica seed - sus scrofa diencephalon - sus scrofa duodenum - sus scrofa gall bladder - sus scrofa pancreas - sus scrofa pylorus - thiamine hydrochloride - trypsin - tryptophan - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-301 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARTEMISIA ABROTANUM FLOWERING TOP (UNII: QG07G580U0) (ARTEMISIA ABROTANUM FLOWERING TOP - UNII:QG07G580U0) ARTEMISIA ABROTANUM FLOWERING TOP 1 [hp_X] in 30 mL PANCRELIPASE AMYLASE (UNII: YOJ58O116E) (PANCRELIPASE AMYLASE - UNII:YOJ58O116E) PANCRELIPASE AMYLASE 2 [hp_X] in 30 mL ANGELICA ARCHANGELICA ROOT (UNII: DTN01M69SN) (ANGELICA ARCHANGELICA ROOT - UNII:DTN01M69SN) ANGELICA ARCHANGELICA ROOT 1 [hp_X] in 30 mL ANISUM (UNII: Q32I0529TM) (ANISUM - UNII:Q32I0529TM) ANISUM 1 [hp_X] in 30 mL ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 2 [hp_X] in 30 mL CITRULLUS COLOCYNTHIS FRUIT PULP (UNII: 23H32AOH17) (CITRULLUS COLOCYNTHIS FRUIT PULP - UNII:23H32AOH17) CITRULLUS COLOCYNTHIS FRUIT PULP 3 [hp_X] in 30 mL SUS SCROFA DIENCEPHALON (UNII: 23PJ4252VL) (SUS SCROFA DIENCEPHALON - UNII:23PJ4252VL) SUS SCROFA DIENCEPHALON 6 [hp_X] in 30 mL SUS SCROFA DUODENUM (UNII: P6J2SFT80O) (SUS SCROFA DUODENUM - UNII:P6J2SFT80O) SUS SCROFA DUODENUM 8 [hp_X] in 30 mL SUS SCROFA GALLBLADDER (UNII: B6A98VOI9I) (SUS SCROFA GALLBLADDER - UNII:B6A98VOI9I) SUS SCROFA GALLBLADDER 8 [hp_X] in 30 mL GENTIANA LUTEA ROOT (UNII: S72O3284MS) (GENTIANA LUTEA ROOT - UNII:S72O3284MS) GENTIANA LUTEA ROOT 1 [hp_X] in 30 mL PORK LIVER (UNII: 6EC706HI7F) (PORK LIVER - UNII:6EC706HI7F) PORK LIVER 8 [hp_X] in 30 mL PANCRELIPASE LIPASE (UNII: 8MYC33932O) (PANCRELIPASE LIPASE - UNII:8MYC33932O) PANCRELIPASE LIPASE 2 [hp_X] in 30 mL MELATONIN (UNII: JL5DK93RCL) (MELATONIN - UNII:JL5DK93RCL) MELATONIN 4 [hp_C] in 30 mL NIACIN (UNII: 2679MF687A) (NIACIN - UNII:2679MF687A) NIACIN 2 [hp_X] in 30 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 3 [hp_X] in 30 mL SUS SCROFA PANCREAS (UNII: 9Y3J3362RY) (SUS SCROFA PANCREAS - UNII:9Y3J3362RY) SUS SCROFA PANCREAS 8 [hp_X] in 30 mL SUS SCROFA PYLORUS (UNII: JHM2AD7V9M) (SUS SCROFA PYLORUS - UNII:JHM2AD7V9M) SUS SCROFA PYLORUS 8 [hp_X] in 30 mL SEROTONIN (UNII: 333DO1RDJY) (SEROTONIN - UNII:333DO1RDJY) SEROTONIN 6 [hp_X] in 30 mL THIAMINE HYDROCHLORIDE (UNII: M572600E5P) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE HYDROCHLORIDE 4 [hp_X] in 30 mL TRYPTOPHAN (UNII: 8DUH1N11BX) (TRYPTOPHAN - UNII:8DUH1N11BX) TRYPTOPHAN 6 [hp_X] in 30 mL TRYPSIN (UNII: GV54A213NN) (TRYPSIN - UNII:GV54A213NN) TRYPSIN 2 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-301-18 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/21/2018 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-301)