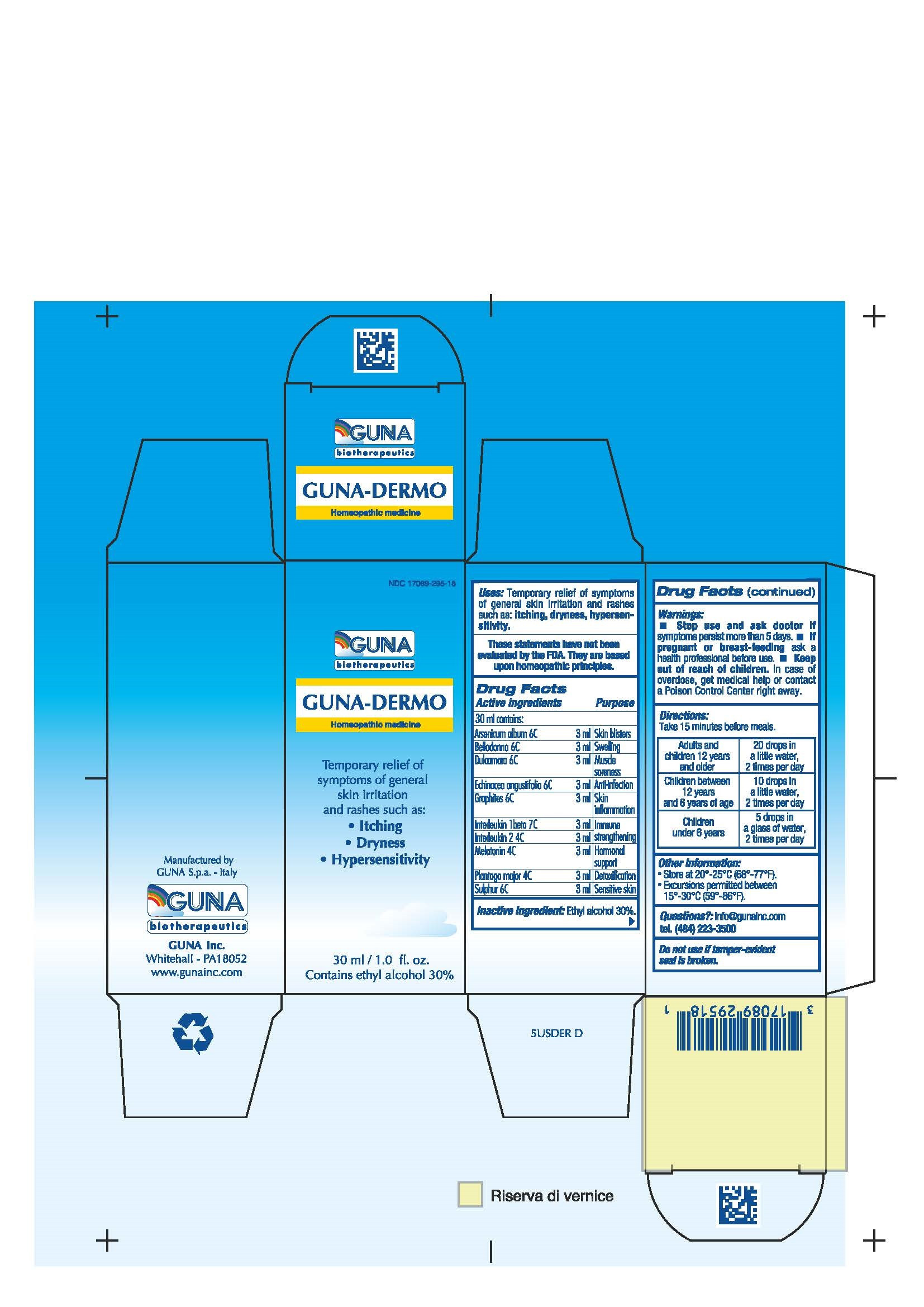
| NDC | 17089-295-18 |
| Set ID | 8bf717eb-fe46-4066-b124-eb715ffc1542 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Guna spa |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENTS/PURPOSE
ARSENICUM ALBUM 6C SKIN BLISTERS
BELLADONNA 6C SWELLING
DULCAMARA 6C MUSCLE SORENESS
ECHINACEA ANGUSTIFOLIA 6C ANTI-INFECTION
GRAPHITES 6C SKIN INFLAMMATION
INTERLEUKIN 1 BETA 7C IMMUNE STRENGTHENING
INTERLEUKIN 2 4C IMMUNE STRENGTHENING
MELATONIN 4C HORMONAL SUPPORT
PLANTAGO MAJOR 4C DETOXIFICATION
SULPHUR 6C SENSITIVE SKIN - USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-DERMO
aldesleukin - arsenic trioxide - atropa belladonna - canakinumab - echinacea angustifolia - graphite - melatonin - plantago major - solanum dulcamara flower - sulfur - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-295 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALDESLEUKIN (UNII: M89N0Q7EQR) (ALDESLEUKIN - UNII:M89N0Q7EQR) ALDESLEUKIN 4 [hp_C] in 30 mL ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 6 [hp_C] in 30 mL ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 6 [hp_C] in 30 mL CANAKINUMAB (UNII: 37CQ2C7X93) (CANAKINUMAB - UNII:37CQ2C7X93) CANAKINUMAB 7 [hp_C] in 30 mL ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 6 [hp_C] in 30 mL GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 6 [hp_C] in 30 mL MELATONIN (UNII: JL5DK93RCL) (MELATONIN - UNII:JL5DK93RCL) MELATONIN 4 [hp_C] in 30 mL PLANTAGO MAJOR (UNII: W2469WNO6U) (PLANTAGO MAJOR - UNII:W2469WNO6U) PLANTAGO MAJOR 4 [hp_C] in 30 mL SOLANUM DULCAMARA FLOWER (UNII: W6J1279A6K) (SOLANUM DULCAMARA FLOWER - UNII:W6J1279A6K) SOLANUM DULCAMARA FLOWER 6 [hp_C] in 30 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 6 [hp_C] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 9 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-295-18 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/23/2006 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-295)