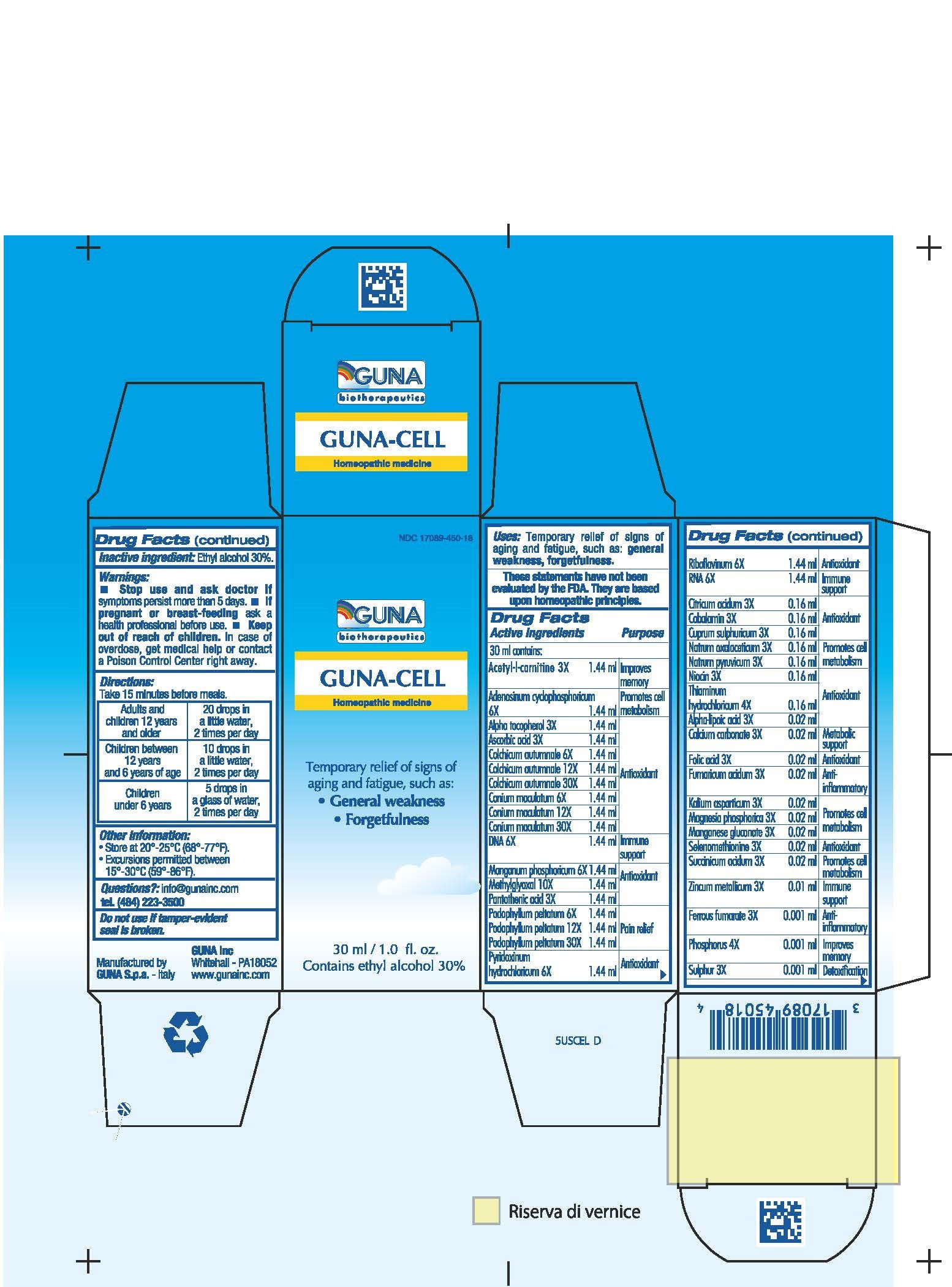
| NDC | 17089-450-18 |
| Set ID | 90db80ed-c037-4e8f-a7f6-304a65f9fe22 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Guna spa |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENTS/PURPOSE
ACETYL-L-CARNITINE 3X IMPROVES MEMORY
ADENOSINUM CYCLOPHOSPHORICUM 6X PROMOTES CELL METABOLISM
A-LIPOICUM ACIDUM 3X ANTIOXIDANT
ALPHA TOCOPHEROL 3X ANTIOXIDANT
ASCORBIC ACID 3X ANTIOXIDANT
CALCIUM CARBONATE 3X METABOLIC SUPPORT
CITRICUM ACIDUM 3X ANTIOXIDANT
COBALAMIN 3X ANTIOXIDANT
COLCHICUM AUTUMNALE 6X 12X 30X ANTIOXIDANT
CONIUM MACULATUM 6X 12X 30X ANTIOXIDANT
CUPRUM SULPHURICUM 3X ANTIOXIDANT
DNA 6X IMMUNE SUPPORT
FERRUM FUMARICUM 3X ANTI-INFLAMMATORY
FOLIC ACID 3X ANTIOXIDANT
FUMARICUM ACIDUM 3X ANTI-INFLAMMATORY
KALIUM ASPARTICUM 3X PROMOTES CELL METABOLISM
MAGNESIA PHOSPHORICA 3X PROMOTES CELL METABOLISM
MANGANESE GLUCONATE 3X PROMOTES CELL METABOLISM
MANGANUM PHOSPHORICUM 6X ANTIOXIDANT
METHYLGLYOXAL 10X ANTIOXIDANT
NATRUM OXALACETICUM 3X PROMOTES CELL METABOLISM
NATRUM PYRUVICUM 3X PROMOTES CELL METABOLISM
NIACIN 3X ANTIOXIDANT
PANTOTHENIC ACID 3X ANTIOXIDANT
PHOSPHORUS 4X IMPROVES MEMORY
PODOPHYLLUM PELTATUM 6X 12X 30X PAIN RELIEF
PYRIDOXINUM HYDROCHLORICUM 6X ANTIOXIDANT
RIBOFLAVINUM 6X ANTIOXIDANT
RNA 6X IMMUNE SUPPORT
SELENOMETHIONINE 3X ANTIOXIDANT
SUCCINICUM ACIDUM 3X PROMOTE CELL METABOLISM
SULPHUR 3X DETOXIFICATION
THIAMINUM HYDROCHLORICUM 4X ANTIOXIDANT
ZINCUM METALLICUM 3X IMMUNE SUPPORT - USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-CELL
acetylcarnitine, l-ampc-alpha-lipoic ac-a-tocopherol-ascorbic acid-calc carb-citric ac-cobalamin-colchicum-conium-cupric sulf-herring sperm dna-ferrous fumarate-folic acid-fumaric acid-kali aspartate-mang phos-mang gluconate-mang phos ii-pyruvaldehyde-nat oxalaceticum-sodium pyruvate-niacin-pantothenic acid-ph-podophyllum peltatum-pyridoxine hydrochloride-riboflavin-saccharomyces cerevisiae rna-selenomethionine-succinic acid-sulfur-thiamine hydrochloride-zinc solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-450 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETYLCARNITINE (UNII: 6DH1W9VH8Q) (LEVOCARNITINE - UNII:0G389FZZ9M) ACETYLCARNITINE 3 [hp_X] in 30 mL ADENOSINE CYCLIC PHOSPHATE (UNII: E0399OZS9N) (ADENOSINE CYCLIC PHOSPHATE - UNII:E0399OZS9N) ADENOSINE CYCLIC PHOSPHATE 6 [hp_X] in 30 mL .ALPHA.-LIPOIC ACID (UNII: 73Y7P0K73Y) (.ALPHA.-LIPOIC ACID - UNII:73Y7P0K73Y) .ALPHA.-LIPOIC ACID 3 [hp_X] in 30 mL .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 3 [hp_X] in 30 mL ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 3 [hp_X] in 30 mL CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB, CARBONATE ION - UNII:7UJQ5OPE7D) CALCIUM CARBONATE 3 [hp_X] in 30 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 3 [hp_X] in 30 mL COBALAMIN (UNII: 8406EY2OQA) (COBALAMIN - UNII:8406EY2OQA) COBALAMIN 3 [hp_X] in 30 mL COLCHICUM AUTUMNALE BULB (UNII: 993QHL78E6) (COLCHICUM AUTUMNALE BULB - UNII:993QHL78E6) COLCHICUM AUTUMNALE BULB 6 [hp_X] in 30 mL CONIUM MACULATUM FLOWERING TOP (UNII: Q28R5GF371) (CONIUM MACULATUM FLOWERING TOP - UNII:Q28R5GF371) CONIUM MACULATUM FLOWERING TOP 6 [hp_X] in 30 mL CUPRIC SULFATE (UNII: LRX7AJ16DT) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 3 [hp_X] in 30 mL HERRING SPERM DNA (UNII: 51FI676N6F) (HERRING SPERM DNA - UNII:51FI676N6F) HERRING SPERM DNA 6 [hp_X] in 30 mL FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 3 [hp_X] in 30 mL FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 3 [hp_X] in 30 mL FUMARIC ACID (UNII: 88XHZ13131) (FUMARIC ACID - UNII:88XHZ13131) FUMARIC ACID 3 [hp_X] in 30 mL PYRUVALDEHYDE (UNII: 722KLD7415) (PYRUVALDEHYDE - UNII:722KLD7415) PYRUVALDEHYDE 10 [hp_X] in 30 mL POTASSIUM ASPARTATE (UNII: OC4598NZEQ) (ASPARTIC ACID - UNII:30KYC7MIAI) POTASSIUM ASPARTATE 3 [hp_X] in 30 mL MAGNESIUM PHOSPHATE, TRIBASIC, PENTAHYDRATE (UNII: 453COF7817) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM PHOSPHATE, TRIBASIC, PENTAHYDRATE 3 [hp_X] in 30 mL MANGANESE GLUCONATE (UNII: 9YY2F980SV) (MANGANESE CATION (2+) - UNII:H6EP7W5457) MANGANESE GLUCONATE 3 [hp_X] in 30 mL MANGANESE PHOSPHATE, DIBASIC (UNII: VZ3U1H7Q5B) (MANGANESE CATION (2+) - UNII:H6EP7W5457) MANGANESE PHOSPHATE, DIBASIC 6 [hp_X] in 30 mL SODIUM DIETHYL OXALACETATE (UNII: 6CA025Y4FG) (DIETHYL OXALACETATE - UNII:15S56468G7) SODIUM DIETHYL OXALACETATE 3 [hp_X] in 30 mL SODIUM PYRUVATE (UNII: POD38AIF08) (PYRUVIC ACID - UNII:8558G7RUTR) SODIUM PYRUVATE 3 [hp_X] in 30 mL NIACIN (UNII: 2679MF687A) (NIACIN - UNII:2679MF687A) NIACIN 3 [hp_X] in 30 mL PANTOTHENIC ACID (UNII: 19F5HK2737) (PANTOTHENIC ACID - UNII:19F5HK2737) PANTOTHENIC ACID 3 [hp_X] in 30 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 4 [hp_X] in 30 mL PODOPHYLLUM PELTATUM ROOT (UNII: 2S713A4VP3) (PODOPHYLLUM PELTATUM ROOT - UNII:2S713A4VP3) PODOPHYLLUM PELTATUM ROOT 6 [hp_X] in 30 mL PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 6 [hp_X] in 30 mL RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 6 [hp_X] in 30 mL SACCHAROMYCES CEREVISIAE RNA (UNII: J17GBZ5VGX) (SACCHAROMYCES CEREVISIAE RNA - UNII:J17GBZ5VGX) SACCHAROMYCES CEREVISIAE RNA 6 [hp_X] in 30 mL SELENOMETHIONINE (UNII: 964MRK2PEL) (SELENOMETHIONINE - UNII:964MRK2PEL) SELENOMETHIONINE 3 [hp_X] in 30 mL SUCCINIC ACID (UNII: AB6MNQ6J6L) (SUCCINIC ACID - UNII:AB6MNQ6J6L) SUCCINIC ACID 3 [hp_X] in 30 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 3 [hp_X] in 30 mL THIAMINE HYDROCHLORIDE (UNII: M572600E5P) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE HYDROCHLORIDE 4 [hp_X] in 30 mL ZINC (UNII: J41CSQ7QDS) (ZINC - UNII:J41CSQ7QDS) ZINC 3 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-450-18 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/27/2010 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-450)