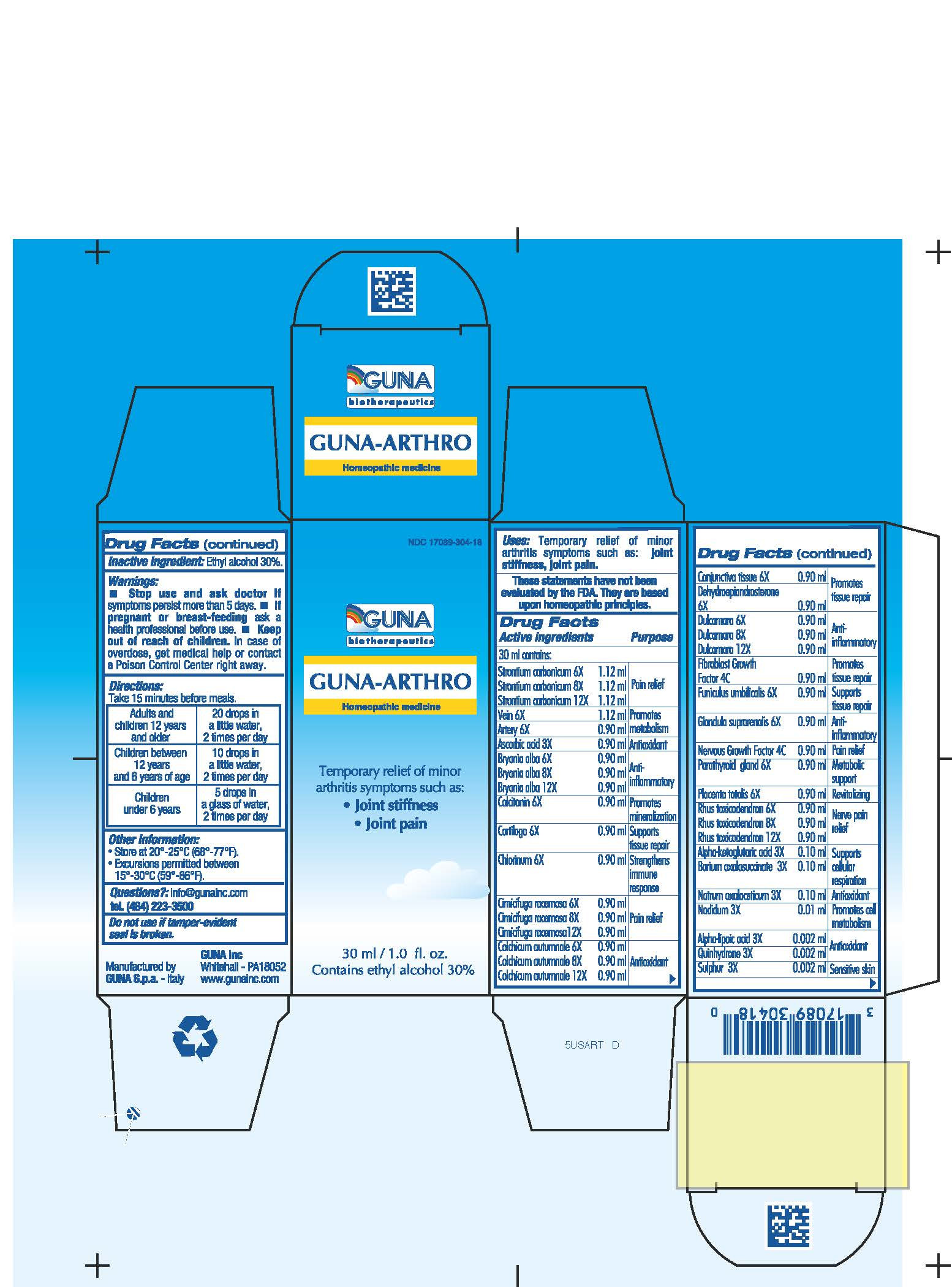
| NDC | 17089-304-18 |
| Set ID | 45ad3f03-0ee8-438f-81e7-3ca772022c98 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Guna spa |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENTS/PURPOSE
ALPHA-KETOGLUTARICUM ACID 3X SUPPORTS CELLULAR RESPIRATION
ALPHA-LIPOIC ACID 3X ANTIOXIDANT
ARTERY 6X PROMOTES METABOLISM
ASCORBIC ACID 3X ANTIOXIDANT
BARIUM OXALOSUCCINATE 3X SUPPORTS CELLULAR RESPIRATION
BRYONIA ALBA 6X, 8X, 12X ANTI-INFLAMMATORY
CALCITONIN 6X PROMOTES MINERALIZATION
CARTILAGO 6X SUPPORTS TISSUE REPAIR
CHLORINUM 6X STRENGTHENS IMMUNE RESPONSE
CIMICIFUGA RACEMOSA 6X, 8X, 12X PAIN RELIEF
COLCHICUM AUTUMNALE 6X, 8X, 12X ANTIOXIDANT
CONJUNCTIVA TISSUE 6X PROMOTES TISSUE REPAIR
DEHYDROEPIANDROSTERONE 6X PROMOTES TISSUE REPAIR
DULCAMARA 6X, 8X, 12X ANTI-INFLAMMATORY
FIBROBLAST GROWTH FACTOR 4C PROMOTES TISSUE REPAIR
FUNICULUS UMBILICALIS 6X SUPPORTS TISSUE REPAIR
GLANDULA SUPRARENALIS 6X ANTI-INFLAMMATORY
NADIDUM 3X PROMOTES CELL METABOLISM
NATRUM OXALACETICUM 3X ANTIOXIDANT
NERVOUS GROWTH FACTOR 4C PAIN RELIEF
PARATHYROID GLAND 6X METABOLIC SUPPORT
PLACENTA TOTALIS 6X REVITALIZING
QUINHYDRONE 3X ANTIOXIDANT
RHUS TOXICODENDRON 6X, 8X, 12X NERVE PAIN RELIEF
STRONTIUM CARBONICUM 6X, 8X, 12X PAIN RELIEF
SULPHUR 3X SENSITIVE SKIN
VEIN 6X PROMOTES METABOLISM - USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-ARTHRO
.alpha.-ketoglutaric acid - alpha lipoic acid - ascorbic acid - barium oxalosuccinate - black cohosh - bryonia alba root - calcitonin human - chlorine - colchicum autumnale bulb - nadide - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-304 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength .ALPHA.-KETOGLUTARIC ACID (UNII: 8ID597Z82X) (.ALPHA.-KETOGLUTARIC ACID - UNII:8ID597Z82X) .ALPHA.-KETOGLUTARIC ACID 3 [hp_X] in 30 mL .ALPHA.-LIPOIC ACID (UNII: 73Y7P0K73Y) (.ALPHA.-LIPOIC ACID - UNII:73Y7P0K73Y) .ALPHA.-LIPOIC ACID 3 [hp_X] in 30 mL SUS SCROFA ARTERY (UNII: 63O327782Q) (SUS SCROFA ARTERY - UNII:63O327782Q) SUS SCROFA ARTERY 6 [hp_X] in 30 mL ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 3 [hp_X] in 30 mL BARIUM OXALOSUCCINATE (UNII: L7A49804ZQ) (BARIUM CATION - UNII:V645272HLN) BARIUM OXALOSUCCINATE 3 [hp_X] in 30 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 6 [hp_X] in 30 mL CALCITONIN HUMAN (UNII: I0IO929019) (CALCITONIN HUMAN - UNII:I0IO929019) CALCITONIN HUMAN 6 [hp_X] in 30 mL SUS SCROFA CARTILAGE (UNII: 73ECW5WG2F) (SUS SCROFA CARTILAGE - UNII:73ECW5WG2F) SUS SCROFA CARTILAGE 6 [hp_X] in 30 mL CHLORINE (UNII: 4R7X1O2820) (CHLORINE - UNII:4R7X1O2820) CHLORINE 6 [hp_X] in 30 mL BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 6 [hp_X] in 30 mL COLCHICUM AUTUMNALE BULB (UNII: 993QHL78E6) (COLCHICUM AUTUMNALE BULB - UNII:993QHL78E6) COLCHICUM AUTUMNALE BULB 6 [hp_X] in 30 mL SUS SCROFA CONJUNCTIVA (UNII: W61ME6Q717) (SUS SCROFA CONJUNCTIVA - UNII:W61ME6Q717) SUS SCROFA CONJUNCTIVA 6 [hp_X] in 30 mL SOLANUM DULCAMARA FLOWER (UNII: W6J1279A6K) (SOLANUM DULCAMARA FLOWER - UNII:W6J1279A6K) SOLANUM DULCAMARA FLOWER 6 [hp_X] in 30 mL VELAFERMIN (UNII: 6Z25C35927) (VELAFERMIN - UNII:6Z25C35927) VELAFERMIN 4 [hp_C] in 30 mL SUS SCROFA UMBILICAL CORD (UNII: 118OYG6W3H) (SUS SCROFA UMBILICAL CORD - UNII:118OYG6W3H) SUS SCROFA UMBILICAL CORD 6 [hp_X] in 30 mL SUS SCROFA ADRENAL GLAND (UNII: 398IYQ16YV) (SUS SCROFA ADRENAL GLAND - UNII:398IYQ16YV) SUS SCROFA ADRENAL GLAND 6 [hp_X] in 30 mL NADIDE (UNII: 0U46U6E8UK) (NADIDE - UNII:0U46U6E8UK) NADIDE 3 [hp_X] in 30 mL SODIUM DIETHYL OXALACETATE (UNII: 6CA025Y4FG) (DIETHYL OXALACETATE - UNII:15S56468G7) SODIUM DIETHYL OXALACETATE 3 [hp_X] in 30 mL NERVE GROWTH FACTOR (UNII: V7FWP1D62O) (NERVE GROWTH FACTOR - UNII:V7FWP1D62O) NERVE GROWTH FACTOR 4 [hp_C] in 30 mL SUS SCROFA PARATHYROID GLAND (UNII: 2KBE35NE8S) (SUS SCROFA PARATHYROID GLAND - UNII:2KBE35NE8S) SUS SCROFA PARATHYROID GLAND 6 [hp_X] in 30 mL SUS SCROFA PLACENTA (UNII: C8CV8867O8) (SUS SCROFA PLACENTA - UNII:C8CV8867O8) SUS SCROFA PLACENTA 6 [hp_X] in 30 mL QUINHYDRONE (UNII: P4A66LQ3QJ) (HYDROQUINONE - UNII:XV74C1N1AE) QUINHYDRONE 3 [hp_X] in 30 mL TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 6 [hp_X] in 30 mL STRONTIUM CARBONATE (UNII: 41YPU4MMCA) (STRONTIUM CATION - UNII:37077S2C93) STRONTIUM CARBONATE 6 [hp_X] in 30 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 3 [hp_X] in 30 mL SUS SCROFA VEIN (UNII: 2510RH3I89) (SUS SCROFA VEIN - UNII:2510RH3I89) SUS SCROFA VEIN 6 [hp_X] in 30 mL PRASTERONE (UNII: 459AG36T1B) (PRASTERONE - UNII:459AG36T1B) PRASTERONE 6 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 9 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-304-18 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/23/2006 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-304)