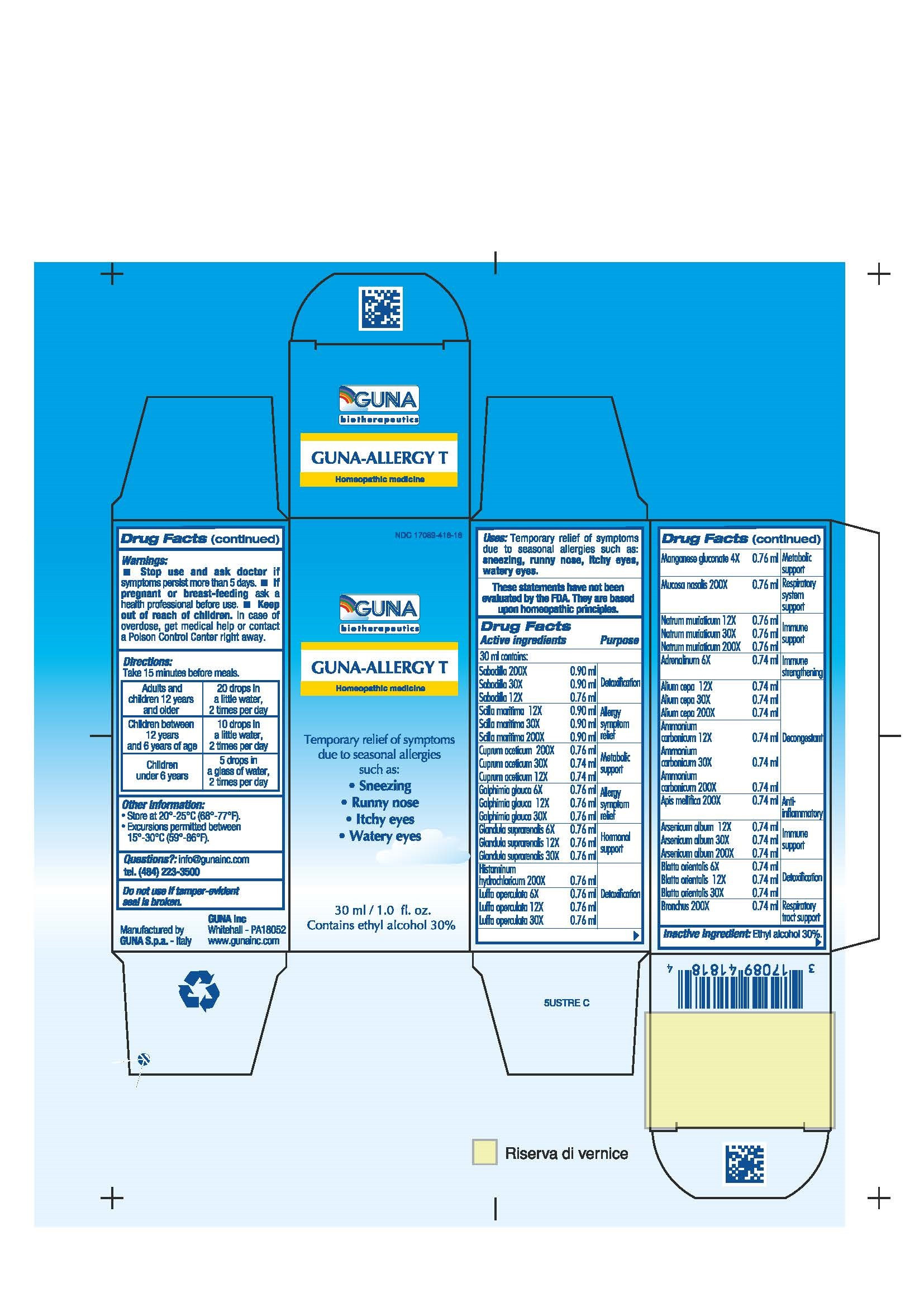
| NDC | 17089-418-18 |
| Set ID | fddba85e-2bec-468f-a01a-b71a3aeed9d3 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Guna spa |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENTS/PURPOSE
ADRENALINUM 6X IMMUNE STRENGTHENING
ALIUM CEPA 12X 30X 200X DECONGESTANT
AMMONIUM CARBONICUM 12X 30X 200X DETOXIFICATION
APIS MELLIFICA 200X ANTI-INFLAMMATORY
ARSENICUM ALBUM 12X 30X 200X IMMUNE SUPPORT
BLATTA ORIENTALIS 6X 12X 30X DETOXIFICATION
BRONCHUS 200X RESPIRATORY TRACT SUPPORT
CUPRUM ACETICUM 12X 30X 200X METABOLIC SUPPORT
GALPHIMIA GLAUCA 6X 12X 30X ALLERGY SYMPTOM RELIEF
GLANDULA SUPRARENALIS 6X 12X 30X HORMONAL SUPPORT
HISTAMINUM HYDROCHLORICUM 200X DETOXIFICATION
LUFFA OPERCULATA 6X 12X 30X DETOXIFICATION
MANGANESE GLUCONATE 4X METABOLIC SUPPORT
MUCOSA NASALIS 200X RESPIRATORY SYSTEM SUPPORT
NATRUM MURIATICUM 12X 30X 200X IMMUNE SUPPORT
SABADILLA 12X 30X 200X DETOXIFICATION
SCILLA MARITIMA 12X 30X 200X ALLERGY SYMPTOM RELIEF - USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-ALLERGY T
ammonium carbonate - apis mellifera - arsenic trioxide - blatta orientalis - cupric acetate - drimia maritima bulb - epinephrine - galphimia glauca flowering top - histamine dihydrochloride - luffa operculata - manganese gluconate - onion - schoenocaulon officinale seed - sodium chloride - sus scrofa adrenal gland - sus scrofa bronchus - sus scrofa nasal mucosa - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-418 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE (UNII: YKH834O4BH) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 6 [hp_X] in 30 mL ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 30 [hp_X] in 30 mL AMMONIUM CARBONATE (UNII: NJ5VT0FKLJ) (AMMONIUM CATION - UNII:54S68520I4) AMMONIUM CARBONATE 30 [hp_X] in 30 mL APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 200 [hp_X] in 30 mL ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 30 [hp_X] in 30 mL BLATTA ORIENTALIS (UNII: 535787266D) (BLATTA ORIENTALIS - UNII:535787266D) BLATTA ORIENTALIS 12 [hp_X] in 30 mL SUS SCROFA BRONCHUS (UNII: K34664156J) (SUS SCROFA BRONCHUS - UNII:K34664156J) SUS SCROFA BRONCHUS 200 [hp_X] in 30 mL CUPRIC ACETATE (UNII: 39M11XPH03) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC ACETATE 30 [hp_X] in 30 mL GALPHIMIA GLAUCA FLOWERING TOP (UNII: 93PH5Q8M7E) (GALPHIMIA GLAUCA FLOWERING TOP - UNII:93PH5Q8M7E) GALPHIMIA GLAUCA FLOWERING TOP 12 [hp_X] in 30 mL SUS SCROFA ADRENAL GLAND (UNII: 398IYQ16YV) (SUS SCROFA ADRENAL GLAND - UNII:398IYQ16YV) SUS SCROFA ADRENAL GLAND 12 [hp_X] in 30 mL HISTAMINE DIHYDROCHLORIDE (UNII: 3POA0Q644U) (HISTAMINE - UNII:820484N8I3) HISTAMINE DIHYDROCHLORIDE 200 [hp_X] in 30 mL LUFFA OPERCULATA FRUIT (UNII: C4MO6809HU) (LUFFA OPERCULATA FRUIT - UNII:C4MO6809HU) LUFFA OPERCULATA FRUIT 12 [hp_X] in 30 mL MANGANESE GLUCONATE (UNII: 9YY2F980SV) (MANGANESE CATION (2+) - UNII:H6EP7W5457) MANGANESE GLUCONATE 4 [hp_X] in 30 mL SUS SCROFA NASAL MUCOSA (UNII: ID3Z1X61WY) (SUS SCROFA NASAL MUCOSA - UNII:ID3Z1X61WY) SUS SCROFA NASAL MUCOSA 200 [hp_X] in 30 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 30 [hp_X] in 30 mL SCHOENOCAULON OFFICINALE SEED (UNII: 6NAF1689IO) (SCHOENOCAULON OFFICINALE SEED - UNII:6NAF1689IO) SCHOENOCAULON OFFICINALE SEED 30 [hp_X] in 30 mL DRIMIA MARITIMA BULB (UNII: 3629601H5D) (DRIMIA MARITIMA BULB - UNII:3629601H5D) DRIMIA MARITIMA BULB 30 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 9 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-418-18 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/27/2010 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-418)