| NDC | 44911-0417-1 |
| Set ID | 4be0c1b3-f854-441b-854e-4c7c929b3fda |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Energique, Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
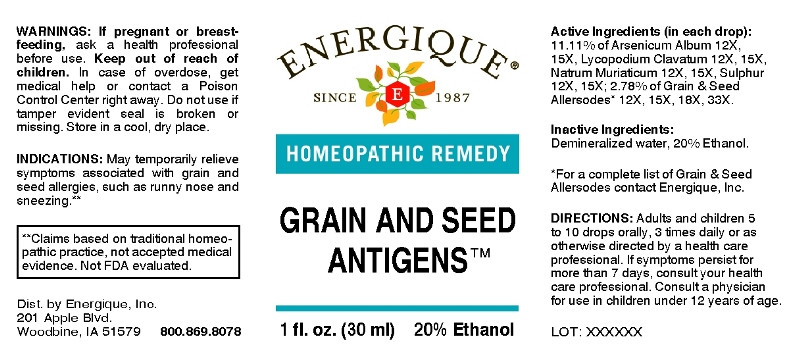
ACTIVE INGREDIENTS:
(in each drop): 11.11% of Arsenicum Album 12X, 15X, Lycopodium Clavatum 12X, 15X, Natrum Muriaticum 12X, 15X, Sulphur 12X, 15X; 2.78% of Bamboo 12X, 15X, 18X, 33X, Barley 12X, 15X, 18X, 33X, Corn 12X, 15X, 18X, 33X, Cotton Seed 12X, 15X, 18X, 33X, Flax Seed (Linum Usitatissimum) 12X, 15X, 18X, 33X, Millet 12X, 15X, 18X, 33X, Milo 12X, 15X, 18X, 33X, Oat 12X, 15X, 18X, 33X, Poppy Seed 12X, 15X, 18X, 33X, Rice 12X, 15X, 18X, 33X, Rye 12X, 15X, 18X, 33X, Safflower 12X, 15X, 18X, 33X, Sesame 12X, 15X, 18X, 33X, Sorghum 12X, 15X, 18X, 33X, Soy Bean 12X, 15X, 18X, 33X, Sugarcane 12X, 15X, 18X, 33X, Sunflower (Helianthus Annuus) 12X, 15X, 18X, 33X, Wheat 12X, 15X, 18X, 33X.
- INDICATIONS:
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INDICATIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
GRAIN AND SEED ANTIGENS
bamboo, barley, corn, cotton seed, flax seed (linum usitatissimum), millet, milo, oat, poppy seed, rice, rye, safflower, sesame, sorghum, soy bean, sugarcane, sunflower (helianthus annuus), wheat, arsenicum album, lycopodium clavatum, natrum muriaticum, sulphur liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:44911-0417 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BAMBUSA VULGARIS TOP (UNII: FIW80T6P6V) (BAMBUSA VULGARIS TOP - UNII:FIW80T6P6V) BAMBUSA VULGARIS TOP 12 [hp_X] in 1 mL BARLEY (UNII: 5PWM7YLI7R) (BARLEY - UNII:5PWM7YLI7R) BARLEY 12 [hp_X] in 1 mL CORN (UNII: 0N8672707O) (CORN - UNII:0N8672707O) CORN 12 [hp_X] in 1 mL COTTON SEED (UNII: DI0ZRJ0MXN) (COTTON SEED - UNII:DI0ZRJ0MXN) COTTON SEED 12 [hp_X] in 1 mL FLAX SEED (UNII: 4110YT348C) (FLAX SEED - UNII:4110YT348C) FLAX SEED 12 [hp_X] in 1 mL MILLET (UNII: TJR6B3R47P) (MILLET - UNII:TJR6B3R47P) MILLET 12 [hp_X] in 1 mL OAT (UNII: Z6J799EAJK) (OAT - UNII:Z6J799EAJK) OAT 12 [hp_X] in 1 mL POPPY SEED (UNII: 60RO23IR87) (POPPY SEED - UNII:60RO23IR87) POPPY SEED 12 [hp_X] in 1 mL BROWN RICE (UNII: 659G217HPG) (BROWN RICE - UNII:659G217HPG) BROWN RICE 12 [hp_X] in 1 mL RYE (UNII: 0R4AQI398X) (RYE - UNII:0R4AQI398X) RYE 12 [hp_X] in 1 mL SAFFLOWER (UNII: 4VBL71TY4Y) (SAFFLOWER - UNII:4VBL71TY4Y) SAFFLOWER 12 [hp_X] in 1 mL SESAME SEED (UNII: 7Y1255HVXR) (SESAME SEED - UNII:7Y1255HVXR) SESAME SEED 12 [hp_X] in 1 mL SORGHUM (UNII: CUL9PN8ELJ) (SORGHUM - UNII:CUL9PN8ELJ) SORGHUM 12 [hp_X] in 1 mL SOYBEAN (UNII: L7HT8F1ZOD) (SOYBEAN - UNII:L7HT8F1ZOD) SOYBEAN 12 [hp_X] in 1 mL SUGARCANE (UNII: 81H2R5AOH3) (SUGARCANE - UNII:81H2R5AOH3) SUGARCANE 12 [hp_X] in 1 mL HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) (HELIANTHUS ANNUUS FLOWERING TOP - UNII:BKJ0J3D1BP) HELIANTHUS ANNUUS FLOWERING TOP 12 [hp_X] in 1 mL WHEAT (UNII: 4J2I0SN84Y) (WHEAT - UNII:4J2I0SN84Y) WHEAT 12 [hp_X] in 1 mL ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 12 [hp_X] in 1 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 12 [hp_X] in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 12 [hp_X] in 1 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44911-0417-1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 02/08/2017 05/28/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/08/2017 05/28/2024 Labeler - Energique, Inc. (789886132) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(44911-0417) , api manufacture(44911-0417) , label(44911-0417) , pack(44911-0417)