| NDC | 10129-001-03, 10129-002-03, 10129-003-03, 10129-004-03, 10129-005-03, 10129-007-02, 10129-008-01, 10129-051-01, 10129-052-01, 10129-053-01, 10129-054-01 |
| Set ID | 759d7479-2d97-09ee-e053-2a91aa0ada9f |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Gingi-Pak a Division of the Belport |
| Generic Name | |
| Product Class | alpha-Adrenergic Agonist, beta-Adrenergic Agonist |
| Product Number | |
| Application Number |
- Active ingredient
- Ask a doctor
- Do not use
- Keep out of reach of children
- Pregnancy warning
- Purpose
- Storage
- Precautions
- Inactive ingredient
- Uses- Gingi-Pak Z-Twist
- Direction
- For topical use only
- Uses- Gingi-Pak Soft-Twist
- Uses- Gingi-Pak 2-ply
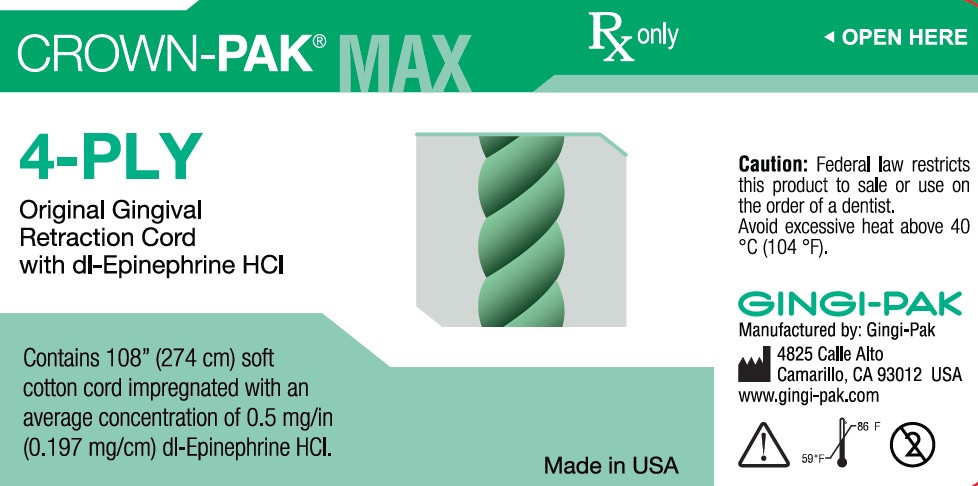
- Uses- Crown-Pak 4-ply
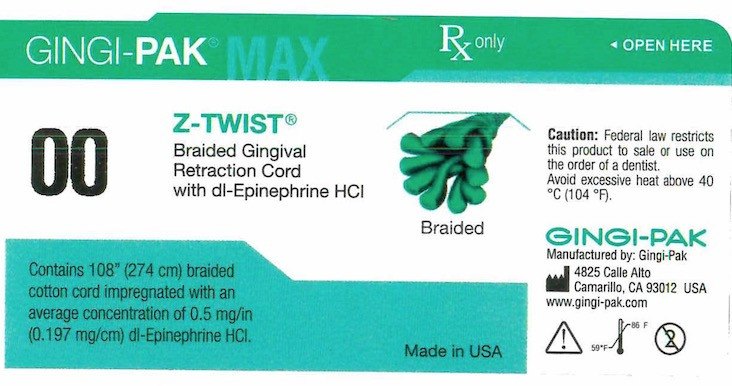
- Principal display- Gingi-Pak No 00
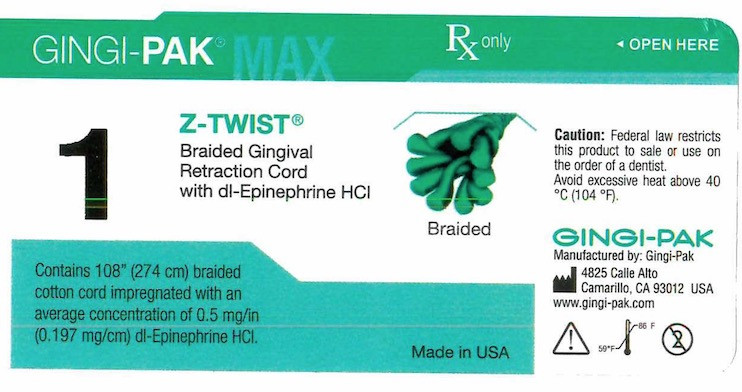
- Principal display- Gingi-Pak No 1
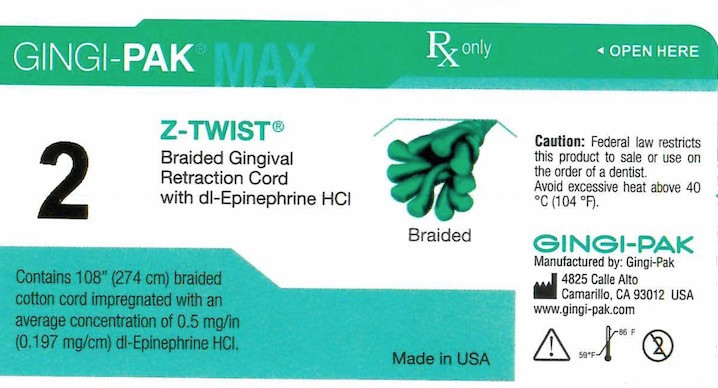
- Principal display- Gingi-Pak No 2
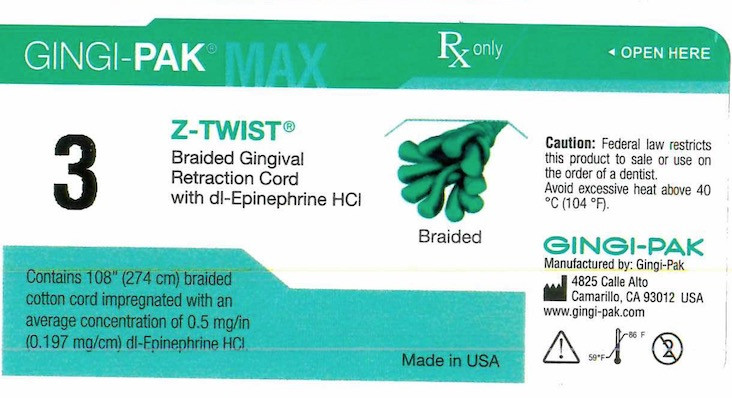
- Principal display- Gingi-Pak No 3
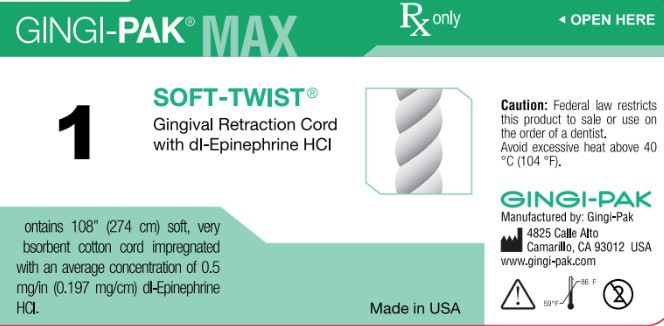
- Principal display-Soft-Twist NO1
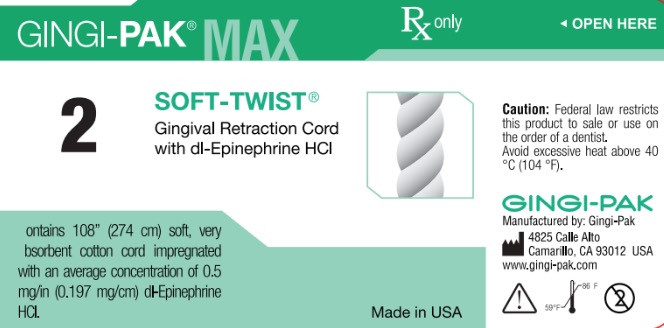
- Principal display-Soft-Twist NO2
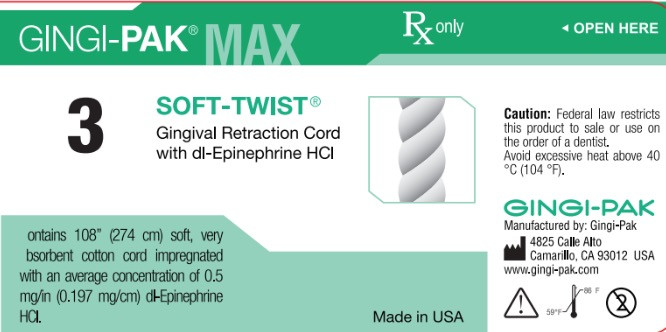
- Principal display-Soft-Twist NO3
- Principal display-2-ply
- Principal display-4-ply
- Principal display-Gingi-Pak Cotton Coil
- Principal display-Gingi-Pak Pellets
-
INGREDIENTS AND APPEARANCE
GINGI-PAK MAX SOFT-TWIST NO 1
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-001 Route of Administration DENTAL, SUBGINGIVAL, PERIODONTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.197 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-001-03 2740 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/26/1987 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/26/1987 GINGI-PAK MAX SOFT-TWIST NO 2
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-002 Route of Administration PERIODONTAL, DENTAL, SUBGINGIVAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.197 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-002-03 2740 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/11/1985 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/11/1985 GINGI-PAK MAX Z-TWIST NO 1
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-052 Route of Administration DENTAL, SUBGINGIVAL, PERIODONTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.197 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-052-01 2740 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/24/1995 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/24/1995 GINGI-PAK MAX Z-TWIST NO 2
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-053 Route of Administration DENTAL, SUBGINGIVAL, PERIODONTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.197 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-053-01 2740 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/24/1995 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/24/1995 GINGI-PAK MAX Z-TWIST NO 3
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-054 Route of Administration SUBGINGIVAL, DENTAL, PERIODONTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.197 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-054-01 2740 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/24/1995 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/24/1995 GINGI-PAK MAX 2-PLY
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-004 Route of Administration PERIODONTAL, DENTAL, SUBGINGIVAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.197 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-004-03 2740 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/29/1984 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/29/1984 GINGI-PAK MAX Z-TWIST NO 00
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-051 Route of Administration DENTAL, SUBGINGIVAL, PERIODONTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.197 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-051-01 2740 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/24/1995 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/24/1995 GINGI-PAK MAX SOFT-TWIST NO 3
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-003 Route of Administration PERIODONTAL, DENTAL, SUBGINGIVAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.197 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-003-03 2740 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/11/1985 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/11/1985 CROWN-PAK MAX 4-PLY
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-005 Route of Administration PERIODONTAL, DENTAL, SUBGINGIVAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.197 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-005-03 2740 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/29/1984 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/29/1984 GINGI-PAK COTTON COIL
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-008 Route of Administration PERIODONTAL, DENTAL, SUBGINGIVAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 1.18 mg in 10 mm Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) 10 mm in 10 mm SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 6.15 ug in 10 mm EDETIC ACID (UNII: 9G34HU7RV0) 0.033 ug in 10 mm METHYLPARABEN (UNII: A2I8C7HI9T) 1.65 ug in 10 mm PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.813 ug in 10 mm SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.13 ug in 10 mm SODIUM THIOSULFATE (UNII: HX1032V43M) 2.46 ug in 10 mm SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.15 ug in 10 mm Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-008-01 610 mm in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/26/1987 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/26/1987 GINGI-PAK PELLETS
dl epinephrine hci solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10129-007 Route of Administration PERIODONTAL, DENTAL, SUBGINGIVAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE HYDROCHLORIDE (UNII: WBB047OO38) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.7 mg Inactive Ingredients Ingredient Name Strength SODIUM DITHIONITE (UNII: 2K5B8F6ES1) 2.38 ug EDETIC ACID (UNII: 9G34HU7RV0) 0.013 ug METHYLPARABEN (UNII: A2I8C7HI9T) 0.638 ug PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.314 ug SODIUM CHLORIDE (UNII: 451W47IQ8X) 3.14 ug SODIUM THIOSULFATE (UNII: HX1032V43M) 0.95 ug SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 2.38 ug Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10129-007-02 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/26/1987 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/26/1987 Labeler - Gingi-Pak a Division of the Belport (008480121) Registrant - Jeff Nichols (008480121) Establishment Name Address ID/FEI Business Operations Gingi-Pak a Division of the Belport 008480121 manufacture(10129-051, 10129-052, 10129-053, 10129-054, 10129-001, 10129-002, 10129-003, 10129-004, 10129-005, 10129-007, 10129-008)