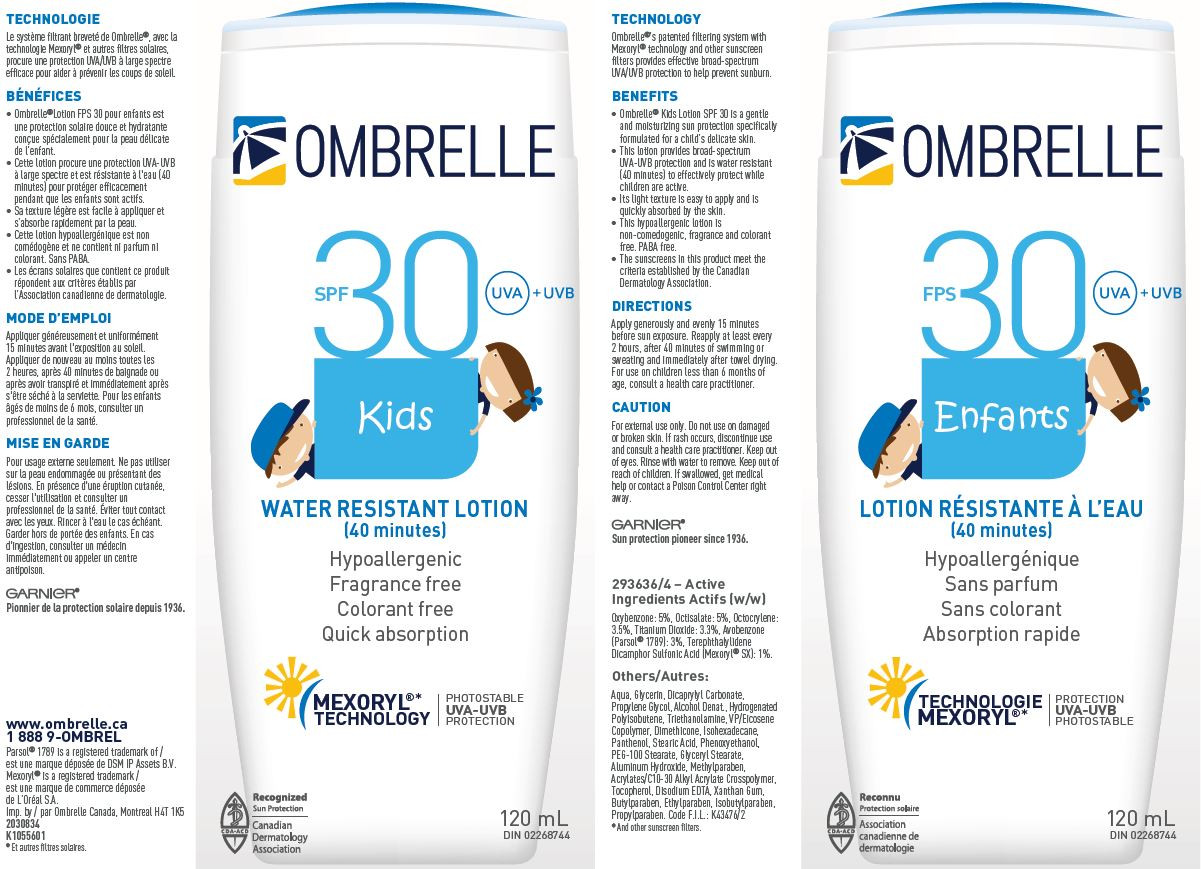
| NDC | 49967-462-01, 49967-462-02 |
| Set ID | a6620063-a357-4b2f-97e5-f0b18930a1ac |
| Category | HUMAN OTC DRUG LABEL |
| Packager | L'Oreal USA Products Inc |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- Active ingredients
- Caution
- Directions
-
Others
Aqua, Glycerin, Dicaprylyl Carbonate, Propylene Glycol, Alcohol Denat., Hydrogenated Polyisobutene, Triethanolanime, VP/Eicosene Copolymer, Dimethicone, Isohexadecane, Panthenol, Stearic Acid, Phenoxyethanol, PEG-100 Stearate, Glyceryl Stearate, Aluminum Hydroxide, Methylparaben, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Tocopherol, Disodium EDTA, Xanthan Gum, Butylparaben, Ethylparaben, Isobutylparaben, Propylparaben
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GARNIER OMBRELLE SPF 30 KIDS WATER RESISTANT
oxybenzone, octisalate, octocrylene, titanium dioxide, avobenzone and terephthalylidene dicamphor sulfonic acid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-462 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 50 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 35 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 33 mg in 1 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL ECAMSULE (UNII: M94R1PM439) (ECAMSULE - UNII:M94R1PM439) ECAMSULE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALCOHOL (UNII: 3K9958V90M) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) TROLAMINE (UNII: 9O3K93S3TK) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) DIMETHICONE (UNII: 92RU3N3Y1O) ISOHEXADECANE (UNII: 918X1OUF1E) PANTHENOL (UNII: WV9CM0O67Z) STEARIC ACID (UNII: 4ELV7Z65AP) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG-100 STEARATE (UNII: YD01N1999R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYLPARABEN (UNII: A2I8C7HI9T) TOCOPHEROL (UNII: R0ZB2556P8) EDETATE DISODIUM (UNII: 7FLD91C86K) XANTHAN GUM (UNII: TTV12P4NEE) BUTYLPARABEN (UNII: 3QPI1U3FV8) ETHYLPARABEN (UNII: 14255EXE39) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-462-01 1 in 1 CARTON 10/12/2011 1 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:49967-462-02 1 in 1 CARTON 10/12/2011 2 240 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date export only 10/12/2011 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations L'Oreal USA, Inc. 624244349 manufacture(49967-462) , pack(49967-462)