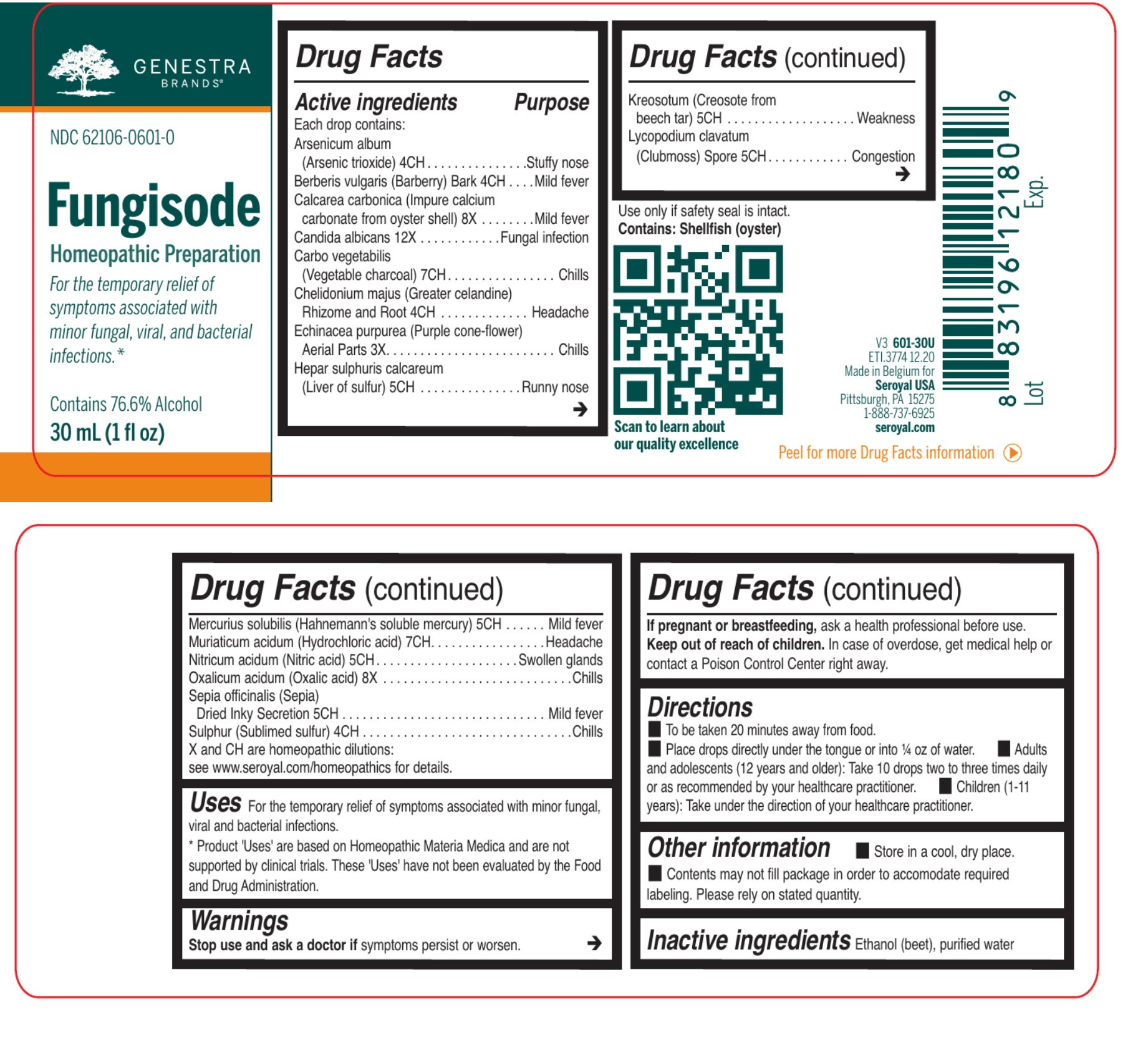
| NDC | 62106-0601-0 |
| Set ID | 55c5dfc4-3757-6ac2-e054-00144ff88e88 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Seroyal USA |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENT
Active ingredients
Each drop contains:
Arsenicum album (Arsenic trioxide) 4CH
Berberis vulgaris (Barberry) Bark 4CH
Calcarea carbonica (Impure calcium carbonate from oyster shell) 8X
Candida albicans 12X
Carbo vegetabilis (Vegetable charcoal) 7CH
Chelidonium majus (Greater celandine) Rhizome and Root 4CH
Echinacea purpurea (Purple cone-flower) Aerial Parts 3X
Hepar sulphuris calcareum (Liver of sulfur) 5CH
Kreosotum (Creosote from beech tar) 5CHLycopodium clavatum (Clubmoss) Spore 5CH
Mercurius solubilis (Hahnemann's soluble mercury) 5CH
Muriaticum acidum (Hydrochloric acid) 7CH
Nitricum acidum (Nitric acid) 5CH
Oxalicum acidum (Oxalic acid) 8X
Sepia officinalis (Sepia) Dried Inky Secretion 5CH
Sulphur (Sublimed sulfur) 4CH - PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
-
DOSAGE & ADMINISTRATION
Directions
To be taken 20 minutes away from food.
Place drops directly under the tongue or into ¼ oz of water.Adults and adolescents (12 years and older):
Take 10 drops two to three times daily or as recommended by your healthcare practitioner.
Children (under 12 years):
Take under the direction of your healthcare practitioner.
-
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms associated with minor fungal, viral and bacterial infections.
Directions
To be taken 20 minutes away from food.
Place drops directly under the tongue or into ¼ oz of water.Adults and adolescents (12 years and older):
Take 10 drops two to three times daily or as recommended by your healthcare practitioner.
Children (under 12 years):
Take under the direction of your healthcare practitioner.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FUNGISODE
arsenicum album, berberis vulgaris, calcarea carbonica, candida albicans, carbo vegetabilis, chelidonium majus, echinacea purpurea, hepar sulphuris calcareum, kreosotum, lycopodium clavatum, mercurius solubilis, muriaticum acidum, nitricum acidum, oxalicum acidum, sepia officinalis, sulphur liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-0601 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 4 [hp_C] in 30 mL OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 8 [hp_X] in 30 mL CANDIDA ALBICANS (UNII: 4D7G21HDBC) (CANDIDA ALBICANS - UNII:4D7G21HDBC) CANDIDA ALBICANS 12 [hp_X] in 30 mL ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 7 [hp_C] in 30 mL NITRIC ACID (UNII: 411VRN1TV4) (NITRIC ACID - UNII:411VRN1TV4) NITRIC ACID 5 [hp_C] in 30 mL WOOD CREOSOTE (UNII: 3JYG22FD73) (WOOD CREOSOTE - UNII:3JYG22FD73) WOOD CREOSOTE 5 [hp_C] in 30 mL MERCURIUS SOLUBILIS (UNII: 324Y4038G2) (MERCURIUS SOLUBILIS - UNII:324Y4038G2) MERCURIUS SOLUBILIS 5 [hp_C] in 30 mL BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 4 [hp_C] in 30 mL ECHINACEA PURPUREA FLOWERING TOP (UNII: 2EMS3QFX65) (ECHINACEA PURPUREA FLOWERING TOP - UNII:2EMS3QFX65) ECHINACEA PURPUREA FLOWERING TOP 3 [hp_X] in 30 mL OXALIC ACID DIHYDRATE (UNII: 0K2L2IJ59O) (OXALIC ACID - UNII:9E7R5L6H31) OXALIC ACID DIHYDRATE 8 [hp_X] in 30 mL SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 5 [hp_C] in 30 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 4 [hp_C] in 30 mL CHELIDONIUM MAJUS ROOT (UNII: FLT36UCF0N) (CHELIDONIUM MAJUS ROOT - UNII:FLT36UCF0N) CHELIDONIUM MAJUS ROOT 4 [hp_C] in 30 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 5 [hp_C] in 30 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 5 [hp_C] in 30 mL HYDROCHLORIC ACID (UNII: QTT17582CB) (HYDROCHLORIC ACID - UNII:QTT17582CB) HYDROCHLORIC ACID 7 [hp_C] in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-0601-0 1 in 1 CARTON 08/02/2017 1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2017 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN'UP 401010287 manufacture(62106-0601)