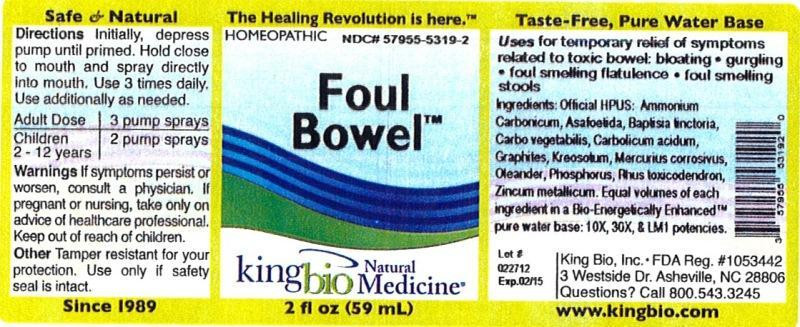
| NDC | 57955-5319-2 |
| Set ID | c177f622-217f-4834-9c4e-fb0f56a19206 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | King Bio Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- Active Ingredients
- Inactive Ingredient
- Purpose
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Directions
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FOUL BOWEL
ammonium carbonicum, asafoetida, baptisia tinctoria, carbo vegetabilis, carbolicum acidum, graphites, kreosotum, mercurius corrosivus, oleander, phosphorus, rhus toxicodendron and zincum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57955-5319 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMMONIUM CARBONATE (UNII: NJ5VT0FKLJ) (AMMONIUM CATION - UNII:54S68520I4) AMMONIUM CARBONATE 10 [hp_X] in 59 mL ASAFETIDA (UNII: W9FZA51AS1) (ASAFETIDA - UNII:W9FZA51AS1) ASAFETIDA 10 [hp_X] in 59 mL BAPTISIA TINCTORIA ROOT (UNII: 5EF0HWI5WU) (BAPTISIA TINCTORIA ROOT - UNII:5EF0HWI5WU) BAPTISIA TINCTORIA ROOT 10 [hp_X] in 59 mL ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 10 [hp_X] in 59 mL PHENOL (UNII: 339NCG44TV) (PHENOL - UNII:339NCG44TV) PHENOL 10 [hp_X] in 59 mL GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 10 [hp_X] in 59 mL WOOD CREOSOTE (UNII: 3JYG22FD73) (WOOD CREOSOTE - UNII:3JYG22FD73) WOOD CREOSOTE 10 [hp_X] in 59 mL MERCURIC CHLORIDE (UNII: 53GH7MZT1R) (MERCURIC CATION - UNII:ED30FJ8Y42) MERCURIC CHLORIDE 10 [hp_X] in 59 mL NERIUM OLEANDER LEAF (UNII: 7KV510R6H6) (NERIUM OLEANDER LEAF - UNII:7KV510R6H6) NERIUM OLEANDER LEAF 10 [hp_X] in 59 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 10 [hp_X] in 59 mL TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 10 [hp_X] in 59 mL ZINC (UNII: J41CSQ7QDS) (ZINC - UNII:J41CSQ7QDS) ZINC 10 [hp_X] in 59 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57955-5319-2 59 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/27/2012 Labeler - King Bio Inc. (617901350) Registrant - King Bio Inc. (617901350) Establishment Name Address ID/FEI Business Operations King Bio Inc. 617901350 api manufacture(57955-5319)