| NDC | 51852-100-01 |
| Set ID | db368653-fce9-4773-af14-08067409529f |
| Category | HUMAN OTC DRUG LABEL |
| Packager | LIFElabs, a Division of Atico International USA, INC. |
| Generic Name | |
| Product Class | Aminoglycoside Antibacterial |
| Product Number | |
| Application Number | PART333B |
- First Aid Triple Antibiotic and Pain Relief (Maximum Strength)
- PURPOSE
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- INDICATIONS & USAGE
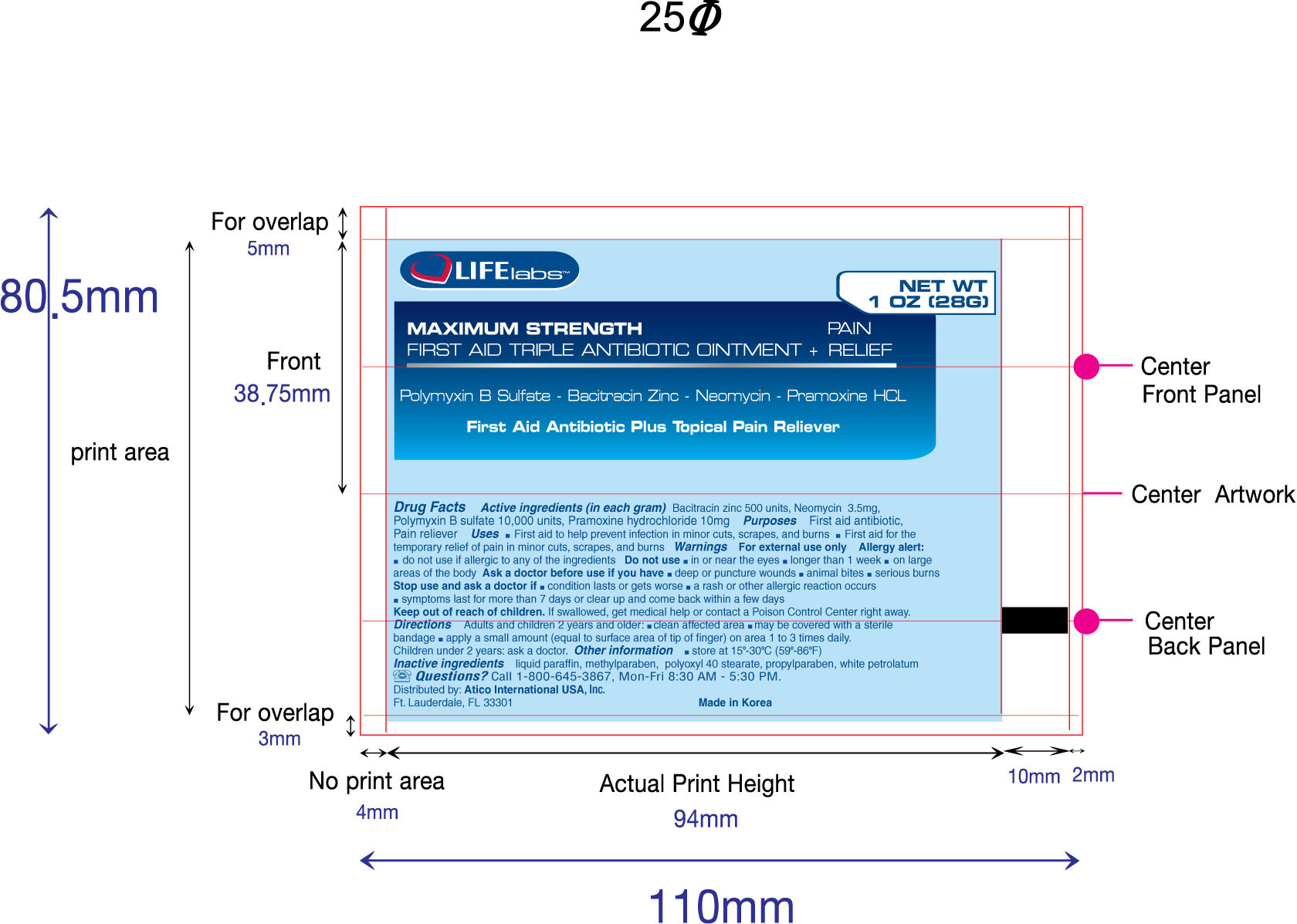
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FIRST AID TRIPLE ANTIBIOTIC AND PAIN RELIEF MAXIMUM STRENGTH
bacitracin zinc, neomycin, polymyxin b sulfate, pramoxine hydrocloride ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51852-100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Bacitracin Zinc (UNII: 89Y4M234ES) (Bacitracin - UNII:58H6RWO52I) Bacitracin Zinc 500 [USP'U] in 1 g Neomycin Sulfate (UNII: 057Y626693) (Neomycin - UNII:I16QD7X297) Neomycin Sulfate 3.5 mg in 1 g Polymyxin B Sulfate (UNII: 19371312D4) (Polymyxin B - UNII:J2VZ07J96K) Polymyxin B Sulfate 10000 [USP'U] in 1 g Pramoxine Hydrochloride (UNII: 88AYB867L5) (Pramoxine - UNII:068X84E056) Pramoxine Hydrochloride 10 mg in 1 g Inactive Ingredients Ingredient Name Strength Mineral Oil (UNII: T5L8T28FGP) Methylparaben (UNII: A2I8C7HI9T) Polyoxyl 40 Stearate (UNII: 13A4J4NH9I) Propylparaben (UNII: Z8IX2SC1OH) Petrolatum (UNII: 4T6H12BN9U) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51852-100-01 1 in 1 CARTON 1 28 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 11/01/2010 Labeler - LIFElabs, a Division of Atico International USA, INC. (073876450)