| NDC | 71742-0004-1 |
| Set ID | 694f0c36-b656-471a-9f38-2efb5193e05a |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Guangzhou Renuma Medical Systems Co., Ltd |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
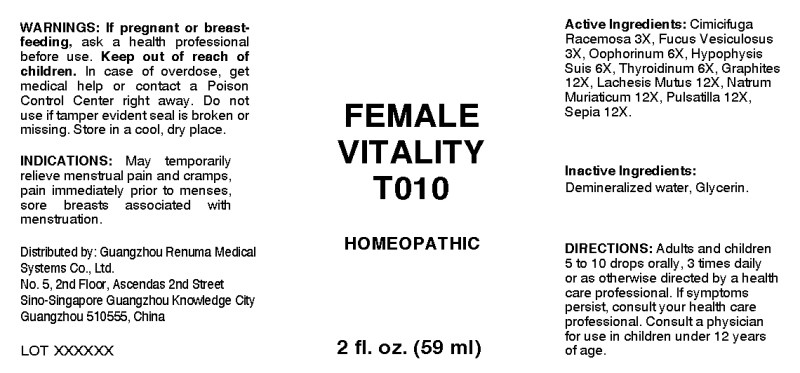
- ACTIVE INGREDIENTS:
- INDICATIONS:
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INDICATIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
FEMALE VITALITY T010
cimicifuga racemosa, fucus vesiculosus, oophorinum (suis), hypophysis suis, thyroidinum (suis), graphites, lachesis mutus, natrum muriaticum, pulsatilla (pratensis), sepia liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71742-0004 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 3 [hp_X] in 1 mL FUCUS VESICULOSUS (UNII: 535G2ABX9M) (FUCUS VESICULOSUS - UNII:535G2ABX9M) FUCUS VESICULOSUS 3 [hp_X] in 1 mL SUS SCROFA OVARY (UNII: S7YTV04R8O) (SUS SCROFA OVARY - UNII:S7YTV04R8O) SUS SCROFA OVARY 6 [hp_X] in 1 mL SUS SCROFA PITUITARY GLAND (UNII: E8S87O660T) (SUS SCROFA PITUITARY GLAND - UNII:E8S87O660T) SUS SCROFA PITUITARY GLAND 6 [hp_X] in 1 mL SUS SCROFA THYROID (UNII: 6RV024OAUQ) (SUS SCROFA THYROID - UNII:6RV024OAUQ) SUS SCROFA THYROID 6 [hp_X] in 1 mL GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 12 [hp_X] in 1 mL LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 12 [hp_X] in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 12 [hp_X] in 1 mL ANEMONE PRATENSIS (UNII: 8E272251DI) (ANEMONE PRATENSIS - UNII:8E272251DI) ANEMONE PRATENSIS 12 [hp_X] in 1 mL SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71742-0004-1 59 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 10/20/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/20/2017 Labeler - Guangzhou Renuma Medical Systems Co., Ltd (544538706) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(71742-0004) , api manufacture(71742-0004) , label(71742-0004) , pack(71742-0004)