| NDC | 42961-090-01, 42961-091-01, 42961-091-02, 42961-092-01, 42961-093-01, 42961-094-01, 42961-094-02 |
| Set ID | d38a90ce-4733-4abb-a2ac-8176273a6515 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Cintas Corporation |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number | PART349 |
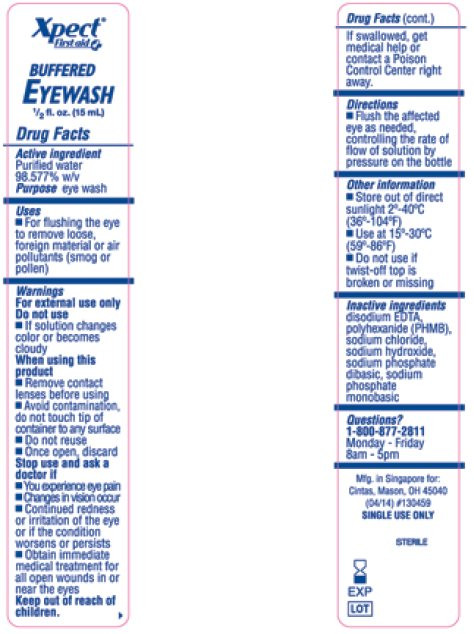
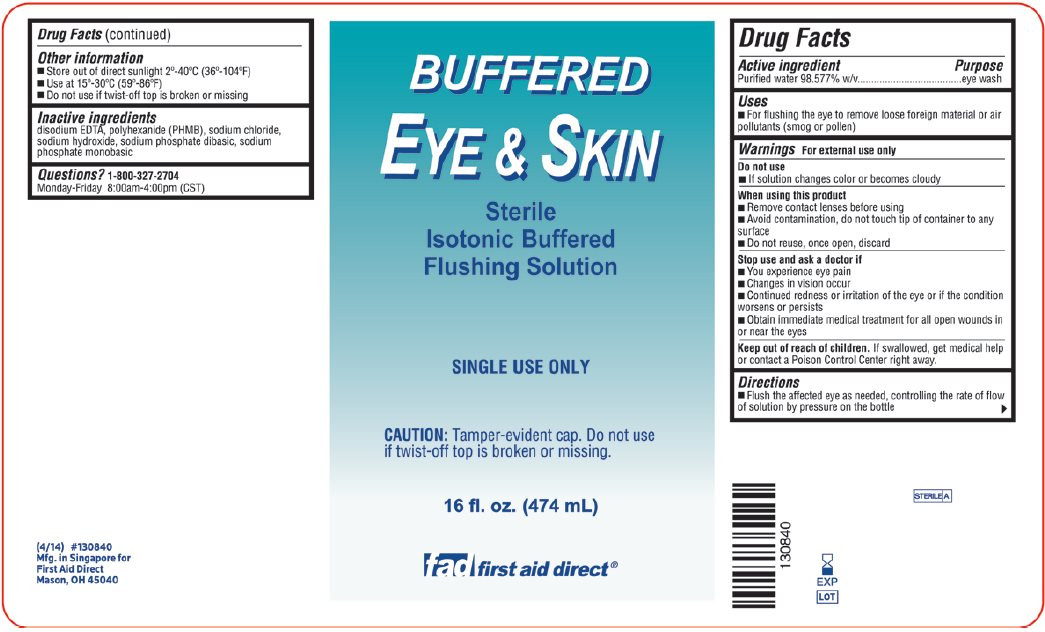
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- •
- If solution changes color or becomes cloudy
When using this product
- •
- Remove contact lenses before using
- •
- Avoid contamination, do not touch tip of container to any surface
- •
- Do not reuse, once open, discard
Stop use and ask a doctor if
- •
- You experience eye pain
- •
- Changes in vision occur
- •
- Continued redness or irritation of the eye or if the condition worsens or persists
- •
- Obtain immediate medical treatment for all open wounds in or near the eyes
- Directions
- Other information
- Inactive ingredients
- Package /Label Principal Display Panel – fad first aid direct Eyewash
- Package/Label Principal Display Panel – Xpect First aid Buffered Eyewash
- Package/Label Principal Display Panel – Eyewash Flushing Solution
- Package/Label Principal Display Panel – Xpect First aid Buffered Eye & Skin
- Package/Label Principal Display Panel – fad first aid direct Buffered Eye & Skin
- Package/Label Principal Display Panel – fad first aid direct Buffered Eye & Skin
-
INGREDIENTS AND APPEARANCE
FAD FIRST AID DIRECT EYEWASH
purified water solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42961-090 Route of Administration OPHTHALMIC, IRRIGATION, TOPICAL, CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 985.77 g in 1000 mL Inactive Ingredients Ingredient Name Strength POLIHEXANIDE (UNII: 322U039GMF) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC, DODECAHYDRATE (UNII: E1W4N241FO) SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42961-090-01 15 mL in 1 VIAL, SINGLE-USE 07/15/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 07/15/2014 XPECT FIRST AID BUFFERED EYEWASH
purified water solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42961-091 Route of Administration OPHTHALMIC, IRRIGATION, TOPICAL, CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 985.77 g in 1000 mL Inactive Ingredients Ingredient Name Strength POLIHEXANIDE (UNII: 322U039GMF) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC, DODECAHYDRATE (UNII: E1W4N241FO) SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42961-091-02 5 in 1 BOX 07/15/2014 1 NDC:42961-091-01 15 mL in 1 VIAL, SINGLE-USE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 07/15/2014 EYEWASH FLUSHING SOLUTION
purified water solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42961-092 Route of Administration OPHTHALMIC, IRRIGATION, TOPICAL, CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 985.77 g in 1000 mL Inactive Ingredients Ingredient Name Strength POLIHEXANIDE (UNII: 322U039GMF) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC, DODECAHYDRATE (UNII: E1W4N241FO) SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42961-092-01 118 mL in 1 BOTTLE, PLASTIC 07/15/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 07/15/2014 XPECT FIRST AID BUFFERED EYE AND SKIN
purified water solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42961-093 Route of Administration OPHTHALMIC, IRRIGATION, TOPICAL, CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 985.77 g in 1000 mL Inactive Ingredients Ingredient Name Strength POLIHEXANIDE (UNII: 322U039GMF) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC, DODECAHYDRATE (UNII: E1W4N241FO) SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42961-093-01 118 mL in 1 BOTTLE, PLASTIC 07/15/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 07/15/2014 FAD FIRST AID DIRECT BUFFERED EYE AND SKIN
purified water solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42961-094 Route of Administration OPHTHALMIC, IRRIGATION, TOPICAL, CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 985.77 g in 1000 mL Inactive Ingredients Ingredient Name Strength POLIHEXANIDE (UNII: 322U039GMF) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC, DODECAHYDRATE (UNII: E1W4N241FO) SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42961-094-01 236 mL in 1 BOTTLE, PLASTIC 07/15/2014 2 NDC:42961-094-02 474 mL in 1 BOTTLE, PLASTIC 07/15/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 07/15/2014 Labeler - Cintas Corporation (056481716)