| NDC | 58414-0001-1, 58414-0002-1, 58414-0003-1, 58414-0004-1, 58414-0005-1, 58414-0006-1, 58414-0007-1, 58414-0008-1, 58414-0009-1, 58414-0010-1 |
| Set ID | 67fc1b45-a7fb-4d6d-a1c2-aa209ef8808d |
| Category | HUMAN OTC DRUG LABEL |
| Packager | NeoStrata Company, Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number | PART352 |
- SPL UNCLASSIFIED SECTION
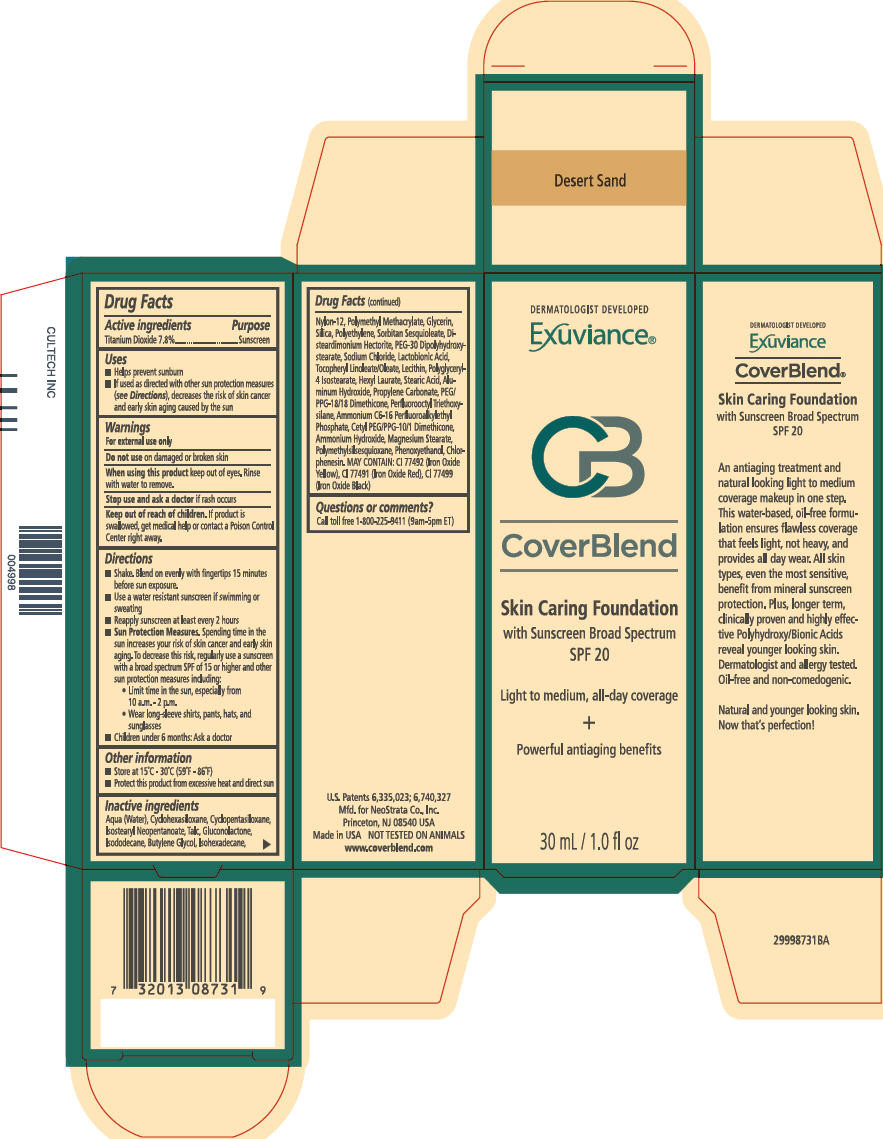
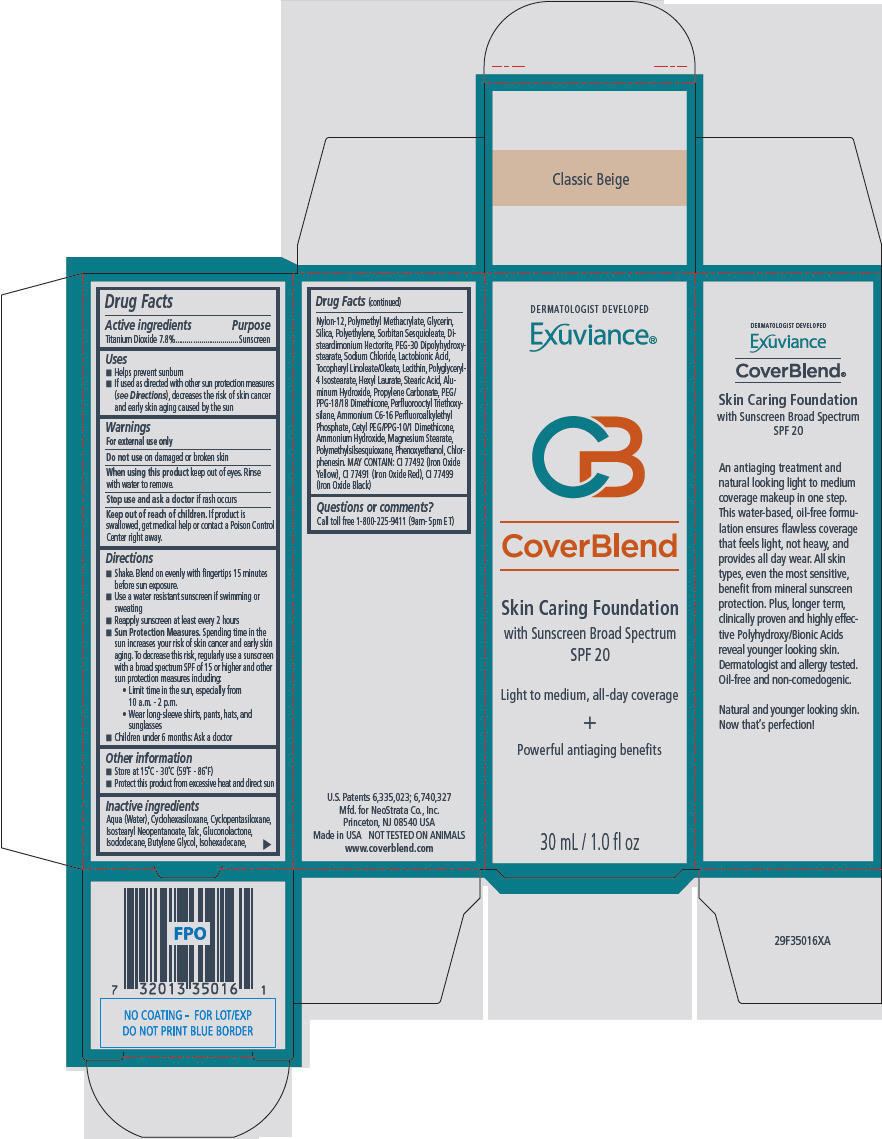
- Active ingredients
- Purpose
-
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- Shake. Blend on evenly with fingertips 15 minutes before sun exposure.
- Use a water resistant sunscreen if swimming or sweating
- Reapply sunscreen at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeve shirts, pants, hats, and sunglasses
- Children under 6 months: Ask a doctor
- Other information
-
Inactive ingredients
Aqua (Water), Cyclohexasiloxane, Cyclopentasiloxane, Isostearyl Neopentanoate, Talc, Gluconolactone, Isododecane, Butylene Glycol, Isohexadecane, Nylon-12, Polymethyl Methacrylate, Glycerin, Silica, Polyethylene, Sorbitan Sesquioleate, Disteardimonium Hectorite, PEG-30 Dipolyhydroxystearate, Sodium Chloride, Lactobionic Acid, Tocopheryl Linoleate/Oleate, Lecithin, Polyglyceryl-4 Isostearate, Hexyl Laurate, Stearic Acid, Aluminum Hydroxide, Propylene Carbonate, PEG/PPG-18/18 Dimethicone, Perfluorooctyl Triethoxysilane, Ammonium C6-16 Perfluoroalkylethyl Phosphate, Cetyl PEG/PPG-10/1 Dimethicone, Ammonium Hydroxide, Magnesium Stearate, Polymethylsilsesquioxane, Phenoxyethanol, Chlorphenesin. MAY CONTAIN: CI 77492 (Iron Oxide Yellow), CI 77491 (Iron Oxide Red), CI 77499 (Iron Oxide Black)
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Bisque
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Blush Beige
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - True Beige
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Neutral Beige
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Warm Beige
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Honey Sand
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Desert Sand
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Classic Beige
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Golden Beige
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Toasted Almond
-
INGREDIENTS AND APPEARANCE
EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 BISQUE
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58414-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 78 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) TALC (UNII: 7SEV7J4R1U) GLUCONOLACTONE (UNII: WQ29KQ9POT) ISODODECANE (UNII: A8289P68Y2) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ISOHEXADECANE (UNII: 918X1OUF1E) NYLON-12 (UNII: 446U8J075B) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PEG-30 DIPOLYHYDROXYSTEARATE (4000 MW) (UNII: 9713Q0S7FO) SODIUM CHLORIDE (UNII: 451W47IQ8X) LACTOBIONIC ACID (UNII: 65R938S4DV) TOCOPHEROL (UNII: R0ZB2556P8) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PERFLUOROOCTYL TRIETHOXYSILANE (UNII: 814P46684U) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) AMMONIA (UNII: 5138Q19F1X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58414-0001-1 1 in 1 CARTON 07/01/2015 08/12/2022 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 07/01/2015 08/12/2022 EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 BLUSH BEIGE
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58414-0002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 78 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) TALC (UNII: 7SEV7J4R1U) GLUCONOLACTONE (UNII: WQ29KQ9POT) ISODODECANE (UNII: A8289P68Y2) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ISOHEXADECANE (UNII: 918X1OUF1E) NYLON-12 (UNII: 446U8J075B) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PEG-30 DIPOLYHYDROXYSTEARATE (4000 MW) (UNII: 9713Q0S7FO) SODIUM CHLORIDE (UNII: 451W47IQ8X) LACTOBIONIC ACID (UNII: 65R938S4DV) TOCOPHEROL (UNII: R0ZB2556P8) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PERFLUOROOCTYL TRIETHOXYSILANE (UNII: 814P46684U) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) AMMONIA (UNII: 5138Q19F1X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58414-0002-1 1 in 1 CARTON 07/01/2015 08/12/2022 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 07/01/2015 08/12/2022 EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 TRUE BEIGE
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58414-0003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 78 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) TALC (UNII: 7SEV7J4R1U) GLUCONOLACTONE (UNII: WQ29KQ9POT) ISODODECANE (UNII: A8289P68Y2) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ISOHEXADECANE (UNII: 918X1OUF1E) NYLON-12 (UNII: 446U8J075B) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PEG-30 DIPOLYHYDROXYSTEARATE (4000 MW) (UNII: 9713Q0S7FO) SODIUM CHLORIDE (UNII: 451W47IQ8X) LACTOBIONIC ACID (UNII: 65R938S4DV) TOCOPHEROL (UNII: R0ZB2556P8) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PERFLUOROOCTYL TRIETHOXYSILANE (UNII: 814P46684U) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) AMMONIA (UNII: 5138Q19F1X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58414-0003-1 1 in 1 CARTON 07/01/2015 08/12/2022 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 07/01/2015 08/12/2022 EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 NEUTRAL BEIGE
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58414-0004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 78 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) TALC (UNII: 7SEV7J4R1U) GLUCONOLACTONE (UNII: WQ29KQ9POT) ISODODECANE (UNII: A8289P68Y2) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ISOHEXADECANE (UNII: 918X1OUF1E) NYLON-12 (UNII: 446U8J075B) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PEG-30 DIPOLYHYDROXYSTEARATE (4000 MW) (UNII: 9713Q0S7FO) SODIUM CHLORIDE (UNII: 451W47IQ8X) LACTOBIONIC ACID (UNII: 65R938S4DV) TOCOPHEROL (UNII: R0ZB2556P8) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PERFLUOROOCTYL TRIETHOXYSILANE (UNII: 814P46684U) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) AMMONIA (UNII: 5138Q19F1X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58414-0004-1 1 in 1 CARTON 07/01/2015 08/12/2022 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 07/01/2015 08/12/2022 EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 WARM BEIGE
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58414-0005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 78 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) TALC (UNII: 7SEV7J4R1U) GLUCONOLACTONE (UNII: WQ29KQ9POT) ISODODECANE (UNII: A8289P68Y2) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ISOHEXADECANE (UNII: 918X1OUF1E) NYLON-12 (UNII: 446U8J075B) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PEG-30 DIPOLYHYDROXYSTEARATE (4000 MW) (UNII: 9713Q0S7FO) SODIUM CHLORIDE (UNII: 451W47IQ8X) LACTOBIONIC ACID (UNII: 65R938S4DV) TOCOPHEROL (UNII: R0ZB2556P8) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PERFLUOROOCTYL TRIETHOXYSILANE (UNII: 814P46684U) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) AMMONIA (UNII: 5138Q19F1X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58414-0005-1 1 in 1 CARTON 07/01/2015 08/12/2022 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 07/01/2015 08/12/2022 EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 HONEY SAND
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58414-0006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 78 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) TALC (UNII: 7SEV7J4R1U) GLUCONOLACTONE (UNII: WQ29KQ9POT) ISODODECANE (UNII: A8289P68Y2) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ISOHEXADECANE (UNII: 918X1OUF1E) NYLON-12 (UNII: 446U8J075B) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PEG-30 DIPOLYHYDROXYSTEARATE (4000 MW) (UNII: 9713Q0S7FO) SODIUM CHLORIDE (UNII: 451W47IQ8X) LACTOBIONIC ACID (UNII: 65R938S4DV) TOCOPHEROL (UNII: R0ZB2556P8) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PERFLUOROOCTYL TRIETHOXYSILANE (UNII: 814P46684U) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) AMMONIA (UNII: 5138Q19F1X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58414-0006-1 1 in 1 CARTON 07/01/2015 08/12/2022 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 07/01/2015 08/12/2022 EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 DESERT SAND
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58414-0007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 78 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) TALC (UNII: 7SEV7J4R1U) GLUCONOLACTONE (UNII: WQ29KQ9POT) ISODODECANE (UNII: A8289P68Y2) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ISOHEXADECANE (UNII: 918X1OUF1E) NYLON-12 (UNII: 446U8J075B) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PEG-30 DIPOLYHYDROXYSTEARATE (4000 MW) (UNII: 9713Q0S7FO) SODIUM CHLORIDE (UNII: 451W47IQ8X) LACTOBIONIC ACID (UNII: 65R938S4DV) TOCOPHEROL (UNII: R0ZB2556P8) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PERFLUOROOCTYL TRIETHOXYSILANE (UNII: 814P46684U) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) AMMONIA (UNII: 5138Q19F1X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58414-0007-1 1 in 1 CARTON 07/01/2015 08/12/2022 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 07/01/2015 08/12/2022 EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 CLASSIC BEIGE
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58414-0008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 78 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) TALC (UNII: 7SEV7J4R1U) GLUCONOLACTONE (UNII: WQ29KQ9POT) ISODODECANE (UNII: A8289P68Y2) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ISOHEXADECANE (UNII: 918X1OUF1E) NYLON-12 (UNII: 446U8J075B) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PEG-30 DIPOLYHYDROXYSTEARATE (4000 MW) (UNII: 9713Q0S7FO) SODIUM CHLORIDE (UNII: 451W47IQ8X) LACTOBIONIC ACID (UNII: 65R938S4DV) TOCOPHEROL (UNII: R0ZB2556P8) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PERFLUOROOCTYL TRIETHOXYSILANE (UNII: 814P46684U) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) AMMONIA (UNII: 5138Q19F1X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58414-0008-1 1 in 1 CARTON 07/01/2015 08/12/2022 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 07/01/2015 08/12/2022 EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 GOLDEN BEIGE
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58414-0009 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 78 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) TALC (UNII: 7SEV7J4R1U) GLUCONOLACTONE (UNII: WQ29KQ9POT) ISODODECANE (UNII: A8289P68Y2) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ISOHEXADECANE (UNII: 918X1OUF1E) NYLON-12 (UNII: 446U8J075B) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PEG-30 DIPOLYHYDROXYSTEARATE (4000 MW) (UNII: 9713Q0S7FO) SODIUM CHLORIDE (UNII: 451W47IQ8X) LACTOBIONIC ACID (UNII: 65R938S4DV) TOCOPHEROL (UNII: R0ZB2556P8) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PERFLUOROOCTYL TRIETHOXYSILANE (UNII: 814P46684U) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) AMMONIA (UNII: 5138Q19F1X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58414-0009-1 1 in 1 CARTON 07/01/2015 08/12/2022 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 07/01/2015 08/12/2022 EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 TOASTED ALMOND
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58414-0010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 78 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) TALC (UNII: 7SEV7J4R1U) GLUCONOLACTONE (UNII: WQ29KQ9POT) ISODODECANE (UNII: A8289P68Y2) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ISOHEXADECANE (UNII: 918X1OUF1E) NYLON-12 (UNII: 446U8J075B) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PEG-30 DIPOLYHYDROXYSTEARATE (4000 MW) (UNII: 9713Q0S7FO) SODIUM CHLORIDE (UNII: 451W47IQ8X) LACTOBIONIC ACID (UNII: 65R938S4DV) TOCOPHEROL (UNII: R0ZB2556P8) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PERFLUOROOCTYL TRIETHOXYSILANE (UNII: 814P46684U) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) AMMONIA (UNII: 5138Q19F1X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58414-0010-1 1 in 1 CARTON 07/01/2015 08/12/2022 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 07/01/2015 08/12/2022 Labeler - NeoStrata Company, Inc. (605754829) Establishment Name Address ID/FEI Business Operations Kolmar Laboratories 001535103 MANUFACTURE(58414-0001, 58414-0002, 58414-0003, 58414-0004, 58414-0005, 58414-0006, 58414-0007, 58414-0008, 58414-0009, 58414-0010)