| NDC | 70116-001-01, 70116-002-01 |
| Set ID | ac941e78-85e1-4517-99a4-34879d3c9d15 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | BIO EARTH MANUFACTURING (PTY) LTD |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number | PART352 |
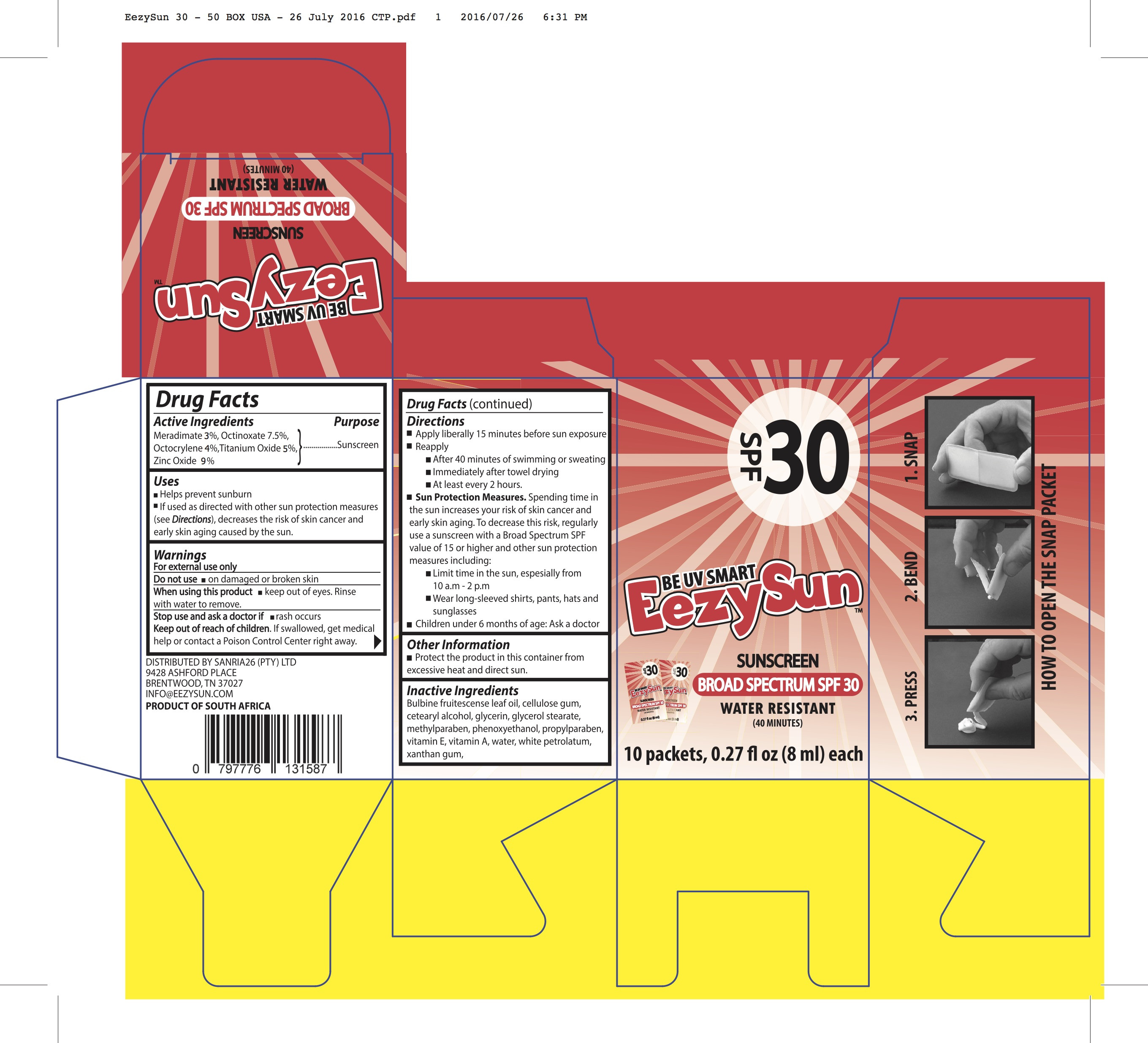
- Actives
- Inactives
-
Dosage and Administration
Directions
Apply liberally 15 minutes before sun exposure Reapply
PLEASE RECYCLE
DISTRIBUTED BY SANRIA26 (PTY) LTD 9428 ASHFORD PLACE BRENTWOOD, TN 37027 INFO@EEZYSUN.COM PRODUCT OF SOUTH AFRICA
After 40 minutes of swimming or sweating Immediately after towel drying
At least every 2 hours.Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
Limit time in the sun, espesially from 10 a.m - 2 p.m
Wear long-sleeved shirts, pants, hats and sunglasses Children under 6 months of age: Ask a docto
- Warnings
- Product Label SPF 30
- Product Label SPF 50
-
INGREDIENTS AND APPEARANCE
EEZY SUN SPF 30
meradimate, octinoxate, octocrylene, titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70116-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MERADIMATE (UNII: J9QGD60OUZ) (MERADIMATE - UNII:J9QGD60OUZ) MERADIMATE 30 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 mL Octocrylene (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) Octocrylene 40 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 50 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 90 mg in 1 mL Inactive Ingredients Ingredient Name Strength BULBINE FRUTESCENS WHOLE (UNII: M2U1C7UW6Y) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) METHYLPARABEN (UNII: A2I8C7HI9T) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLPARABEN (UNII: Z8IX2SC1OH) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) VITAMIN A (UNII: 81G40H8B0T) WATER (UNII: 059QF0KO0R) PETROLATUM (UNII: 4T6H12BN9U) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70116-001-01 8 mL in 1 POUCH; Type 0: Not a Combination Product 12/14/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 12/14/2017 EEZY SUN SPF 50
meradimate, octinoxate, octocrylene, titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70116-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MERADIMATE (UNII: J9QGD60OUZ) (MERADIMATE - UNII:J9QGD60OUZ) MERADIMATE 50 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 mL Octocrylene (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) Octocrylene 80 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 70 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 110 mg in 1 mL Inactive Ingredients Ingredient Name Strength BULBINE FRUTESCENS WHOLE (UNII: M2U1C7UW6Y) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) METHYLPARABEN (UNII: A2I8C7HI9T) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLPARABEN (UNII: Z8IX2SC1OH) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) VITAMIN A (UNII: 81G40H8B0T) WATER (UNII: 059QF0KO0R) PETROLATUM (UNII: 4T6H12BN9U) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70116-002-01 8 mL in 1 POUCH; Type 0: Not a Combination Product 12/14/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 12/14/2017 Labeler - BIO EARTH MANUFACTURING (PTY) LTD (639768436) Establishment Name Address ID/FEI Business Operations BIO EARTH MANUFACTURING (PTY) LTD 639768436 manufacture(70116-001, 70116-002)