| NDC | 57955-7295-2 |
| Set ID | c90c26db-7eff-4038-a112-0b49d415884f |
| Category | HUMAN OTC DRUG LABEL |
| Packager | King Bio Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENT
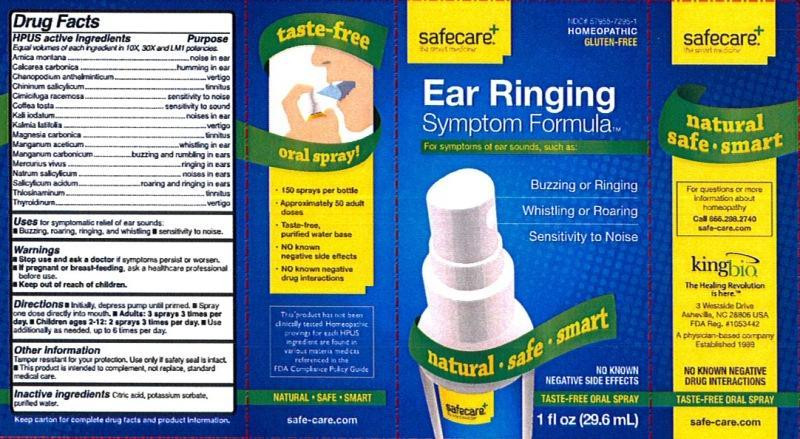
Drug Facts
____________________________________________________________________________________________________________
Equal volumes of each ingredient in 10X, 30X, and LM1 potencies.
HPUS active ingredients: Arnica montana, Calcarea carbonica, Chenopodium anthelminticum, Chininum salicylicum, Cimicifuga racemosa, Coffea tosta, Kali iodatum, Kalmia latifolia, Magnesia carbonica, Manganum aceticum, Manganum carbonicum, Mercurius vivus, Natrum salicylicum, Salicylicum acidum, Thiosinaminum, Thyroidinum.
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
-
PURPOSE
Drug Facts __________________________________________________________________________________________________________________ HPUS active ingredients Purpose
Equal volumes of each ingredient in 10X, 30X, and LM1 potencies.
Arnica montana.....................................................................noise in ear
Calcarea carbonica.................................................................humming in ear
Chenopodium anthelminticum..................................................vertigo
Chininum salicylicum..............................................................tinnitus
Cimicifuga racemosa...............................................................sensitivity to noise
Coffea tosta...........................................................................sensitivity to sound
Kali iodatum..........................................................................noises in ear
Kalmia latifolia.......................................................................vertigo
Magnesia carbonica.................................................................tinnitus
Manganum aceticum...............................................................whistling in ear
Manganum carbonicum...........................................................buzzing and rumbling in ears
Mercurius vivus......................................................................ringing in ears
Natrum salicylicum.................................................................noises in ears
Salicylicum acidum.................................................................roaring and ringing in ears
Thiosinaminum......................................................................tinnitus
Thyroidinum..........................................................................vertigo
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EAR RINGING SYMPTOM FORMULA
arnica montana, calcarea carbonica, chenopodium anthelminticum, chininum salicylicum, cimicifuga racemosa, coffea tosta, kali iodatum, kalmia latifolia, magnesia carbonica, manganum aceticum, manganum carbonicum, mercurius vivus, natrum salicylicum, salicylicum acidum, thiosinaminum, thyroidinum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57955-7295 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 10 [hp_X] in 29.6 mL OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 10 [hp_X] in 29.6 mL DYSPHANIA AMBROSIOIDES (UNII: 4H5RSU087I) (CHENOPODIUM AMBROSIOIDES - UNII:4H5RSU087I) DYSPHANIA AMBROSIOIDES 10 [hp_X] in 29.6 mL QUININE SALICYLATE (UNII: 6DY04L71DR) (SALICYLIC ACID - UNII:O414PZ4LPZ) QUININE SALICYLATE 10 [hp_X] in 29.6 mL BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 10 [hp_X] in 29.6 mL COFFEA ARABICA SEED, ROASTED (UNII: 9H58JRT35E) (COFFEA ARABICA SEED, ROASTED - UNII:9H58JRT35E) COFFEA ARABICA SEED, ROASTED 10 [hp_X] in 29.6 mL POTASSIUM IODIDE (UNII: 1C4QK22F9J) (IODIDE ION - UNII:09G4I6V86Q) IODIDE ION 10 [hp_X] in 29.6 mL KALMIA LATIFOLIA LEAF (UNII: 79N6542N18) (KALMIA LATIFOLIA LEAF - UNII:79N6542N18) KALMIA LATIFOLIA LEAF 10 [hp_X] in 29.6 mL MAGNESIUM CARBONATE (UNII: 0E53J927NA) (CARBONATE ION - UNII:7UJQ5OPE7D) MAGNESIUM CARBONATE 10 [hp_X] in 29.6 mL MANGANESE ACETATE TETRAHYDRATE (UNII: 9TO51D176N) (MANGANESE CATION (2+) - UNII:H6EP7W5457) MANGANESE ACETATE TETRAHYDRATE 10 [hp_X] in 29.6 mL MANGANESE CARBONATE (UNII: 9ZV57512ZM) (MANGANESE CATION (2+) - UNII:H6EP7W5457) MANGANESE CATION (2+) 10 [hp_X] in 29.6 mL MERCURY (UNII: FXS1BY2PGL) (MERCURY - UNII:FXS1BY2PGL) MERCURY 10 [hp_X] in 29.6 mL SODIUM SALICYLATE (UNII: WIQ1H85SYP) (SALICYLIC ACID - UNII:O414PZ4LPZ) SODIUM SALICYLATE 10 [hp_X] in 29.6 mL SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 10 [hp_X] in 29.6 mL ALLYLTHIOUREA (UNII: 706IDJ14B7) (ALLYLTHIOUREA - UNII:706IDJ14B7) ALLYLTHIOUREA 10 [hp_X] in 29.6 mL THYROID, UNSPECIFIED (UNII: 0B4FDL9I6P) (THYROID, UNSPECIFIED - UNII:0B4FDL9I6P) THYROID, UNSPECIFIED 10 [hp_X] in 29.6 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57955-7295-2 1 in 1 CARTON 1 29.6 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/09/2013 Labeler - King Bio Inc. (617901350) Registrant - King Bio Inc. (617901350) Establishment Name Address ID/FEI Business Operations King Bio Inc. 617901350 api manufacture(57955-7295)