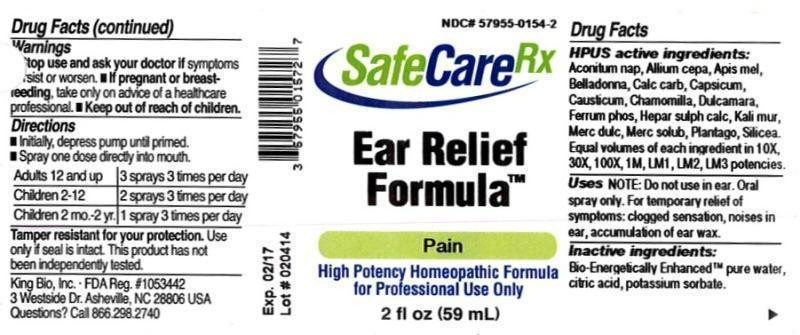
| NDC | 57955-0154-2 |
| Set ID | 60dbafc0-8239-4817-8fa0-243a31b6ba36 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | King Bio Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENT
Drug Facts__________________________________________________________________________________________________________
HPUS active ingredients: Aconitum napellus, Allium cepa, Apis mellifica, Belladonna, Calcarea carbonica, Capsicum annuum, Causticum, Chamomilla, Dulcamara, Ferrum phosphoricum, Hepar sulphuris calcareum, Kali muriaticum, Mercurius dulcis, Mercurius solubilis, Plantago major, Silicea. Equal volumes of each ingredient in 10X, 30X, 100X, 1M, LM1, LM2, LM3 potencies.
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EAR RELIEF FORMULA
aconitum napellus, allium cepa, apis mellifica, belladonna, calcarea carbonica, capsicum annuum, causticum, chamomilla, dulcamara, ferrum phosphoricum, hepar sulphuris calcareum, kali muriaticum, mercurius dulcis, mercurius solubilis, plantago major, silicea liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57955-0154 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 10 [hp_X] in 59 mL ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 10 [hp_X] in 59 mL APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 10 [hp_X] in 59 mL ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 10 [hp_X] in 59 mL OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 10 [hp_X] in 59 mL CAPSICUM (UNII: 00UK7646FG) (CAPSICUM - UNII:00UK7646FG) CAPSICUM 10 [hp_X] in 59 mL CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 10 [hp_X] in 59 mL MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 10 [hp_X] in 59 mL SOLANUM DULCAMARA TOP (UNII: KPS1B1162N) (SOLANUM DULCAMARA TOP - UNII:KPS1B1162N) SOLANUM DULCAMARA TOP 10 [hp_X] in 59 mL FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERROSOFERRIC PHOSPHATE 10 [hp_X] in 59 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 10 [hp_X] in 59 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CATION 10 [hp_X] in 59 mL CALOMEL (UNII: J2D46N657D) (CALOMEL - UNII:J2D46N657D) CALOMEL 10 [hp_X] in 59 mL MERCURIUS SOLUBILIS (UNII: 324Y4038G2) (MERCURIUS SOLUBILIS - UNII:324Y4038G2) MERCURIUS SOLUBILIS 10 [hp_X] in 59 mL PLANTAGO MAJOR (UNII: W2469WNO6U) (PLANTAGO MAJOR - UNII:W2469WNO6U) PLANTAGO MAJOR 10 [hp_X] in 59 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (COLLOIDAL SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 10 [hp_X] in 59 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57955-0154-2 59 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/18/2015 Labeler - King Bio Inc. (617901350) Registrant - King Bio Inc. (617901350) Establishment Name Address ID/FEI Business Operations King Bio Inc. 617901350 manufacture(57955-0154)