| NDC | 52731-7008-1, 52731-7008-2 |
| Set ID | b2f6078f-2680-456c-8bd7-0e55e0d002c2 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Nova Homeopathic Therapeutics, Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- Purpose:
- Usage and Dosage:
- DOSAGE & ADMINISTRATION
- Warnings:
- WARNINGS
- Active Ingredients:
- Inactive Ingredients:
- QUESTIONS
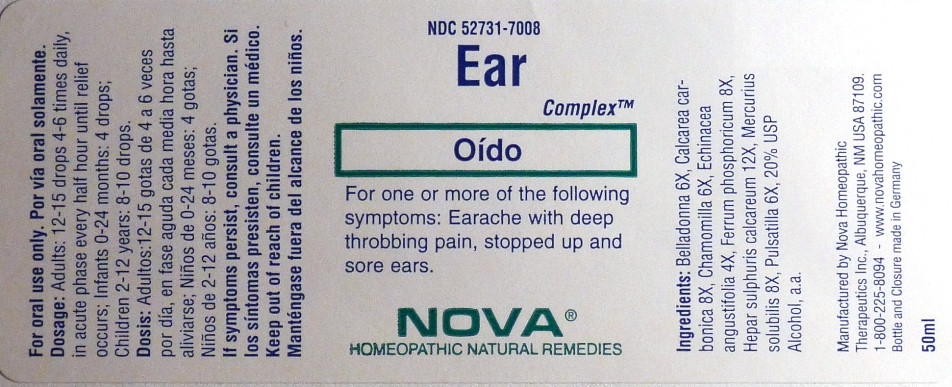
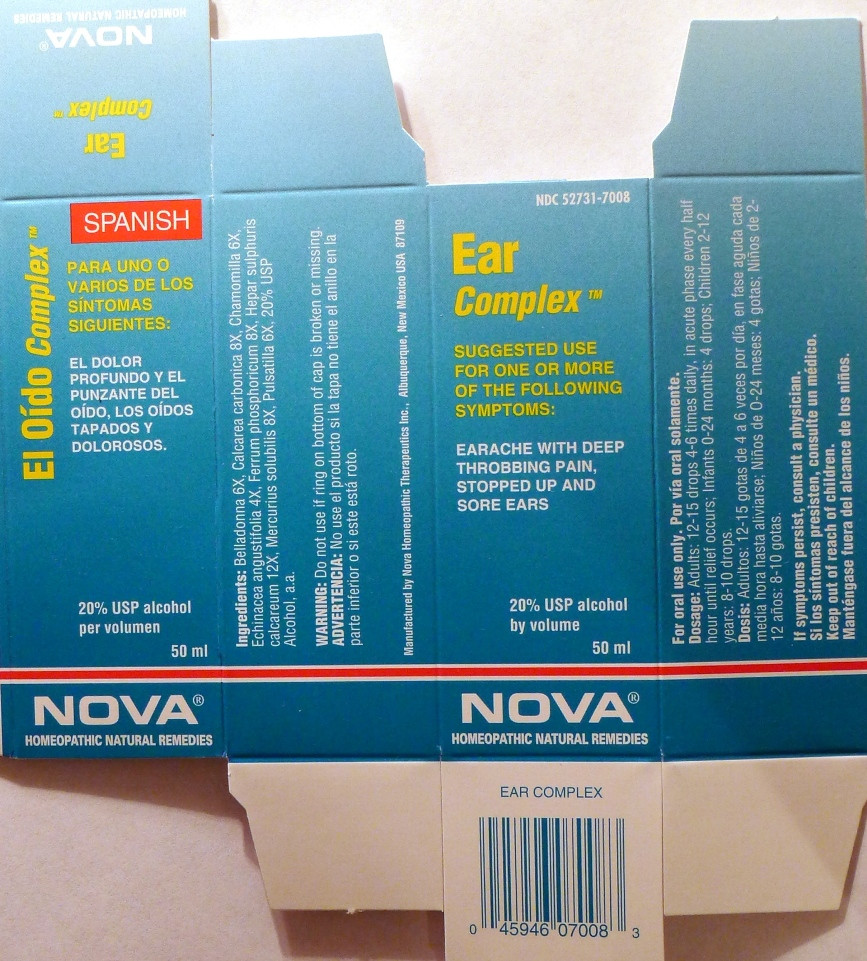
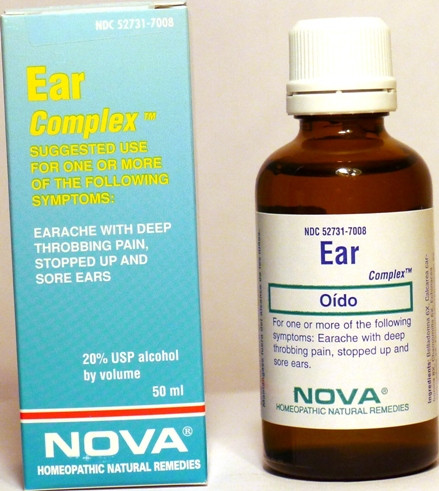
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EAR COMPLEX
belladonna, calcarea carbonica, chamomilla, echinacea angustifolia, ferrum phosphoricum, hepar sulphuris calcareum, mercurius solubilis, pulsatilla liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52731-7008 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 6 [hp_X] in 1 mL OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 8 [hp_X] in 1 mL MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 6 [hp_X] in 1 mL ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 4 [hp_X] in 1 mL FERRUM PHOSPHORICUM (UNII: 91GQH8I5F7) (FERRUM PHOSPHORICUM - UNII:91GQH8I5F7) FERRUM PHOSPHORICUM 8 [hp_X] in 1 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM SULFIDE 8 [hp_X] in 1 mL MERCURIUS SOLUBILIS (UNII: 324Y4038G2) (MERCURIUS SOLUBILIS - UNII:324Y4038G2) MERCURIUS SOLUBILIS 12 [hp_X] in 1 mL PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 6 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52731-7008-2 1 in 1 BOX 1 NDC:52731-7008-1 50 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/01/2011 Labeler - Nova Homeopathic Therapeutics, Inc. (194394540) Registrant - Nova Homeopathic Therapeutics, Inc. (194394540) Establishment Name Address ID/FEI Business Operations Nova Homeopathic Therapeutics, Inc. 194394540 manufacture, label, pack