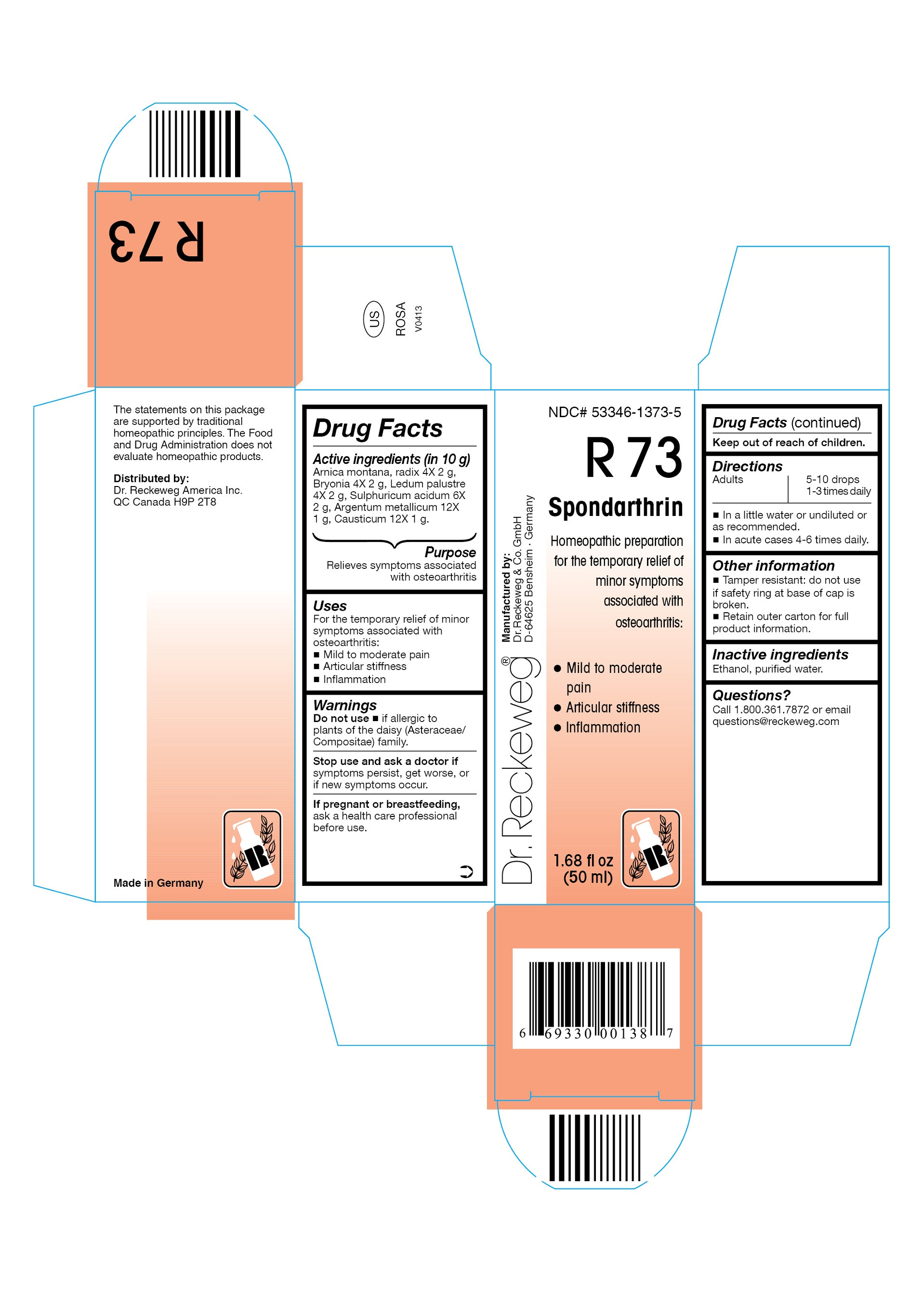
| NDC | 53346-1373-5 |
| Set ID | f9aa006c-af8d-45d6-9804-cf407a6c1efb |
| Category | HUMAN OTC DRUG LABEL |
| Packager | PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DR. RECKEWEG R73 SPONDARTHRIN COMBINATION PRODUCT
arnica montana, radix 4x, bryonia 4x, ledum palustre 4x, sulphuricum acidum 6x, argentum metallicum 12x, causticum 12x liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53346-1373 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA ROOT (UNII: MUE8Y11327) (ARNICA MONTANA ROOT - UNII:MUE8Y11327) ARNICA MONTANA ROOT 4 [hp_X] in 50 mL BRYONIA DIOICA ROOT (UNII: 53UB5FH7CX) (BRYONIA DIOICA ROOT - UNII:53UB5FH7CX) BRYONIA DIOICA ROOT 4 [hp_X] in 50 mL LEDUM PALUSTRE TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) LEDUM PALUSTRE TWIG 4 [hp_X] in 50 mL SULFURIC ACID (UNII: O40UQP6WCF) (SULFURIC ACID - UNII:O40UQP6WCF) SULFURIC ACID 6 [hp_X] in 50 mL SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 50 mL CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 12 [hp_X] in 50 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53346-1373-5 50 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/01/1986 Labeler - PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO (318602612) Establishment Name Address ID/FEI Business Operations PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO 318602612 manufacture(53346-1373)