| NDC | 53346-1349-5 |
| Set ID | c5c2d979-f00b-4b9e-a363-25ac891e3cc4 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
When using this product
- in rare cases, hypersalivation may occur after use, in which case the use of the product should be discontinued
- in very rare cases, skin reactions may occur after use, in which case the product should be discontinued.
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
-
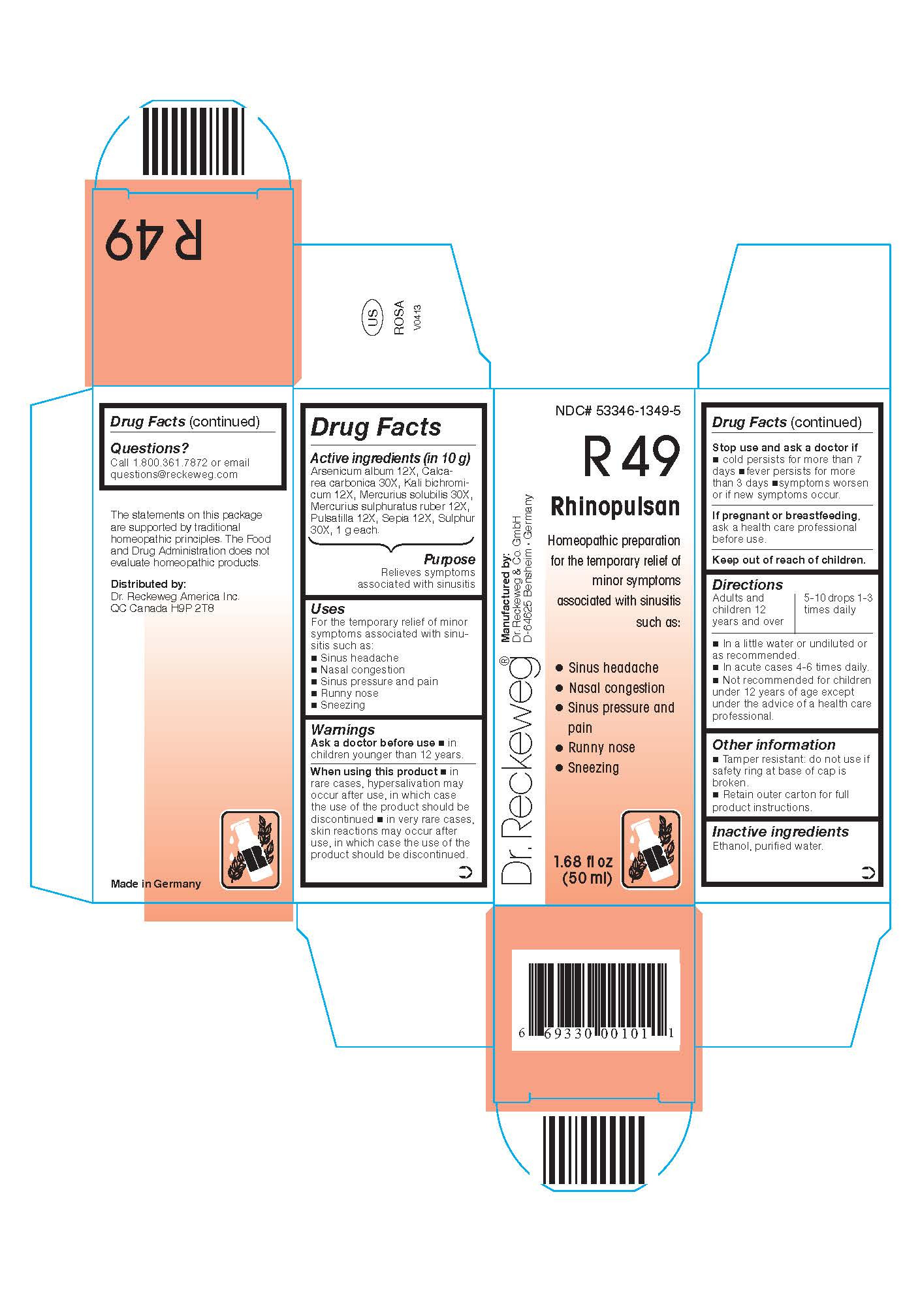
PRINCIPAL DISPLAY PANEL
 NDC# 53346-1349-5
NDC# 53346-1349-5Dr. Reckeweg R49 Rhinopulsan
Homeopathic preparation for the temporary relief of minor symptoms associated with sinusitis such as:
- Sinus headache
- Nasal congestion
- Sinus pressure and pain
- Runny nose
- Sneezing
Manufactured by:
Dr. Reckeweg Co. GmbH
D-64625 Bensheim
Germany
1.68 fl oz
(50 ml) -
INGREDIENTS AND APPEARANCE
DR. RECKEWEG R49 RHINOPULSAN COMBINATION PRODUCT
arsenicum album 12x, calcarea carbonica 30x, kali bichromicum 12x, mercurius solubilis 30x, mercurius sulphuratus ruber 12x, pulsatilla 12x, sepia 12x, sulphur 30x liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53346-1349 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 12 [hp_X] in 50 mL OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 30 [hp_X] in 50 mL POTASSIUM DICHROMATE (UNII: T4423S18FM) (DICHROMATE ION - UNII:9LKY4BFN2V) POTASSIUM DICHROMATE 12 [hp_X] in 50 mL MERCURIUS SOLUBILIS (UNII: 324Y4038G2) (MERCURIUS SOLUBILIS - UNII:324Y4038G2, MERCURIC CATION - UNII:ED30FJ8Y42) MERCURIUS SOLUBILIS 30 [hp_X] in 50 mL MERCURIC SULFIDE (UNII: ZI0T668SF1) (MERCURIC CATION - UNII:ED30FJ8Y42) MERCURIC SULFIDE 12 [hp_X] in 50 mL PULSATILLA PRATENSIS (UNII: 8E272251DI) (PULSATILLA PRATENSIS - UNII:8E272251DI) PULSATILLA PRATENSIS 12 [hp_X] in 50 mL SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 12 [hp_X] in 50 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 30 [hp_X] in 50 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53346-1349-5 50 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/01/1986 Labeler - PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO (318602612) Establishment Name Address ID/FEI Business Operations PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO 318602612 manufacture(53346-1349)