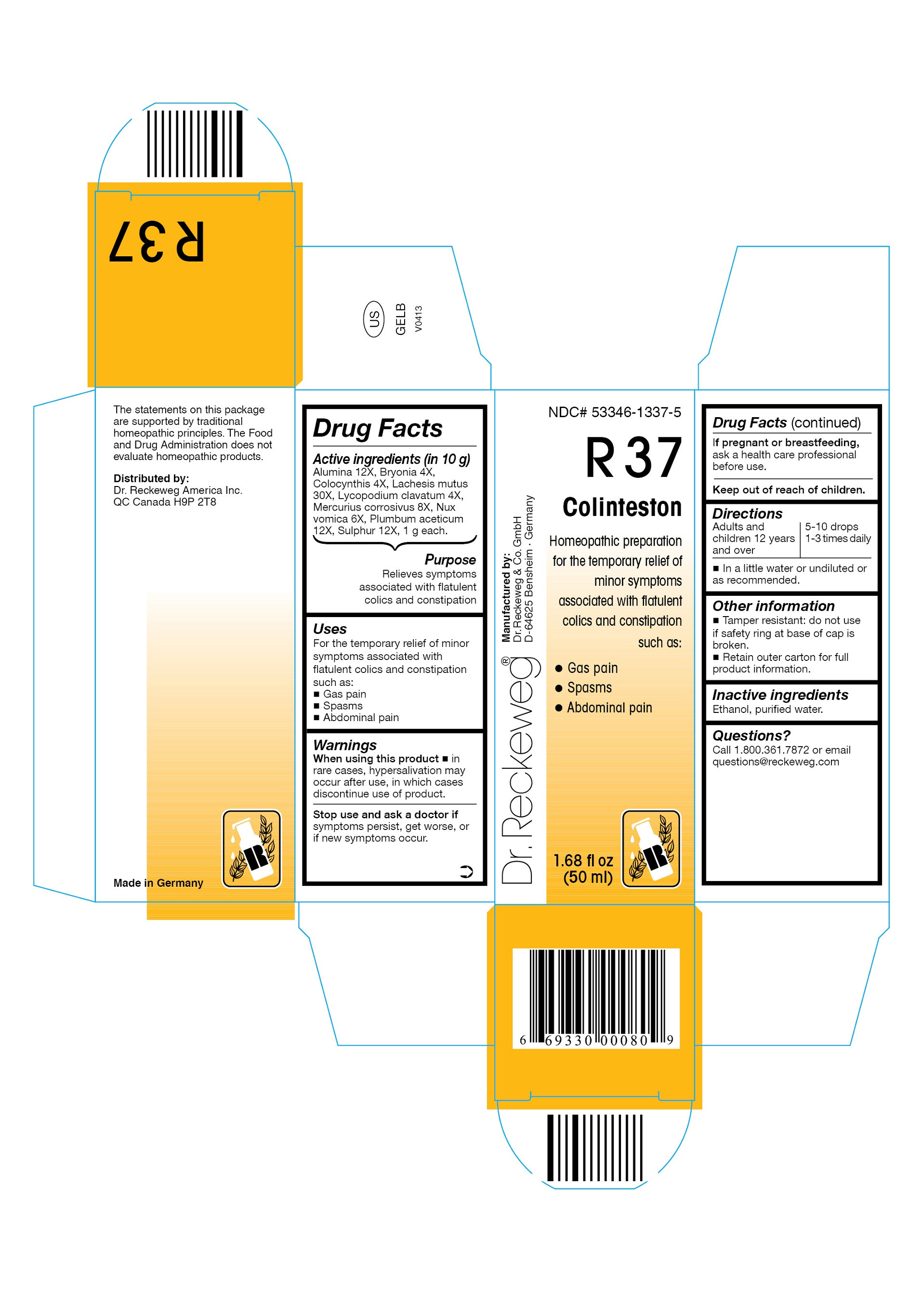
| NDC | 53346-1337-5 |
| Set ID | adde35b0-c6bf-47f2-bffb-7eb0d4318c13 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DR. RECKEWEG R37 COLINTESTON COMBINATION PRODUCT
alumina 12x, bryonia 4x, colocynthis 4x, lachesis mutus 30x, lycopodium clavatum 4x, mercurius corrosivus 8x, nux vomica 6x, plumbum aceticum 12x, sulphur 12x liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53346-1337 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM OXIDE (UNII: LMI26O6933) (ALUMINUM OXIDE - UNII:LMI26O6933) ALUMINUM OXIDE 12 [hp_X] in 50 mL BRYONIA DIOICA ROOT (UNII: 53UB5FH7CX) (BRYONIA CRETICA SUBSP. DIOICA ROOT - UNII:53UB5FH7CX) BRYONIA DIOICA ROOT 4 [hp_X] in 50 mL CITRULLUS COLOCYNTHIS FRUIT PULP (UNII: 23H32AOH17) (CITRULLUS COLOCYNTHIS FRUIT PULP - UNII:23H32AOH17) CITRULLUS COLOCYNTHIS FRUIT PULP 4 [hp_X] in 50 mL LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 30 [hp_X] in 50 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 4 [hp_X] in 50 mL MERCURIC CHLORIDE (UNII: 53GH7MZT1R) (MERCURIC CATION - UNII:ED30FJ8Y42) MERCURIC CHLORIDE 8 [hp_X] in 50 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 6 [hp_X] in 50 mL LEAD ACETATE (UNII: RX077P88RY) (LEAD - UNII:2P299V784P) LEAD ACETATE 12 [hp_X] in 50 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 12 [hp_X] in 50 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53346-1337-5 50 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/01/1986 Labeler - PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO (318602612) Establishment Name Address ID/FEI Business Operations PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO 318602612 manufacture(53346-1337)