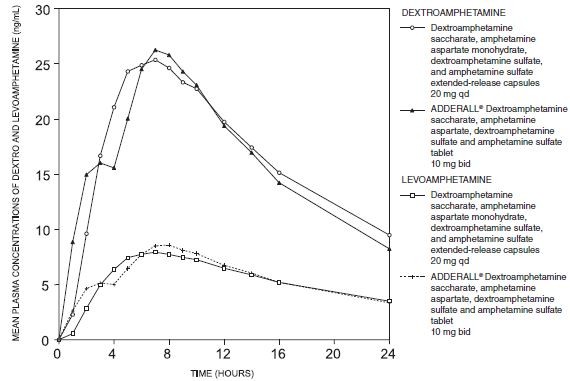
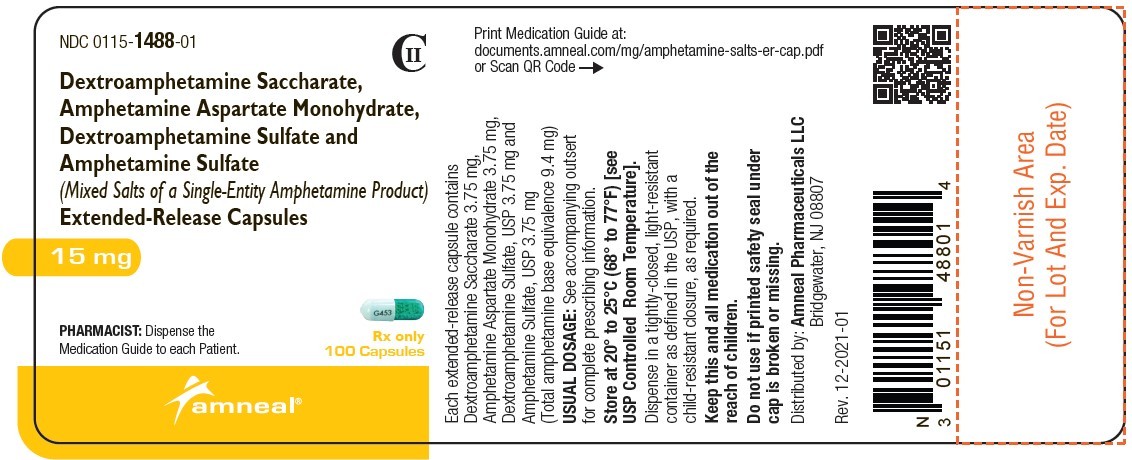
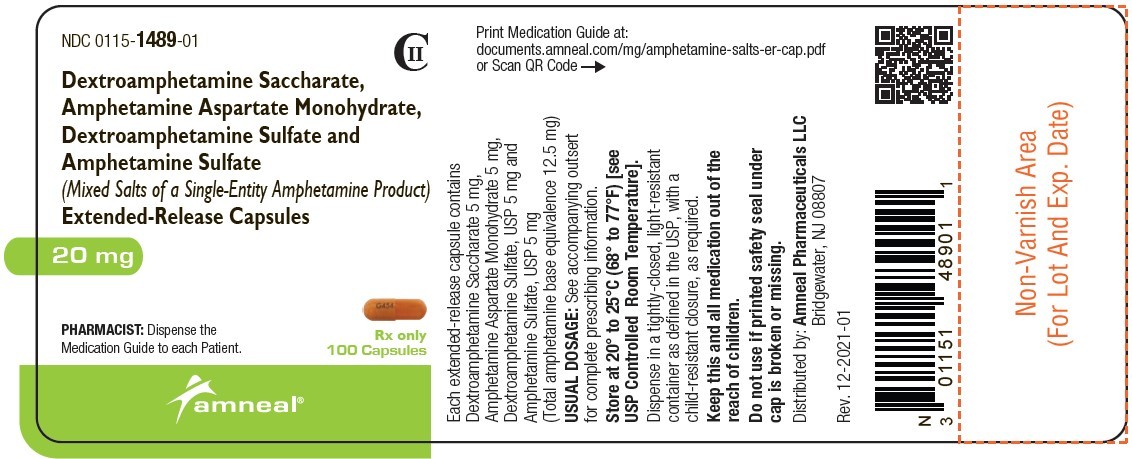
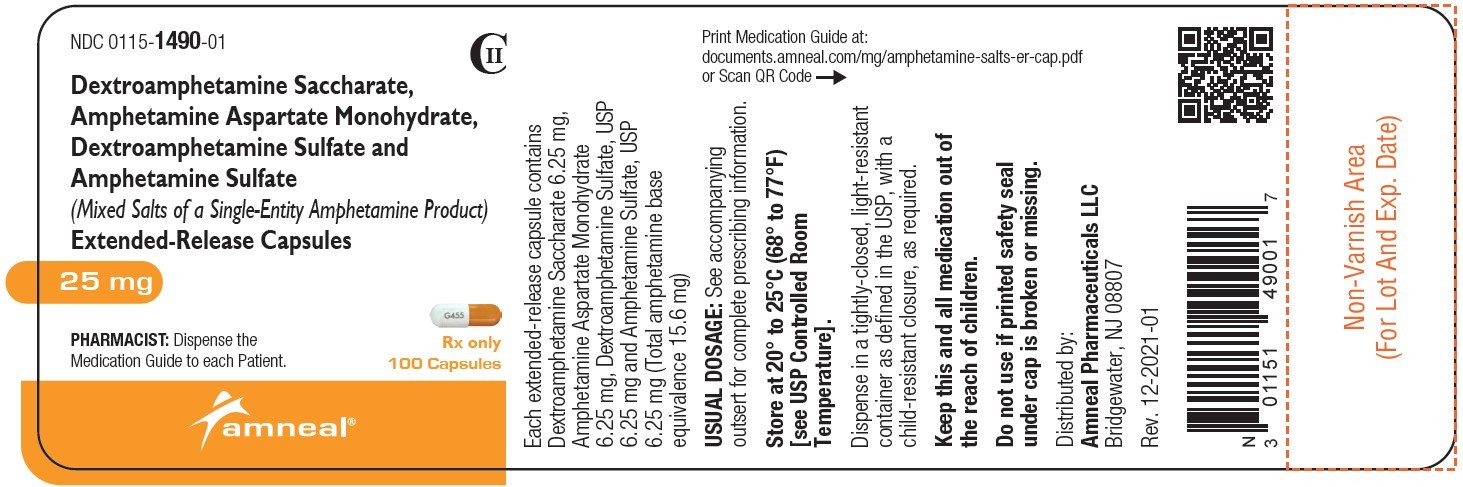
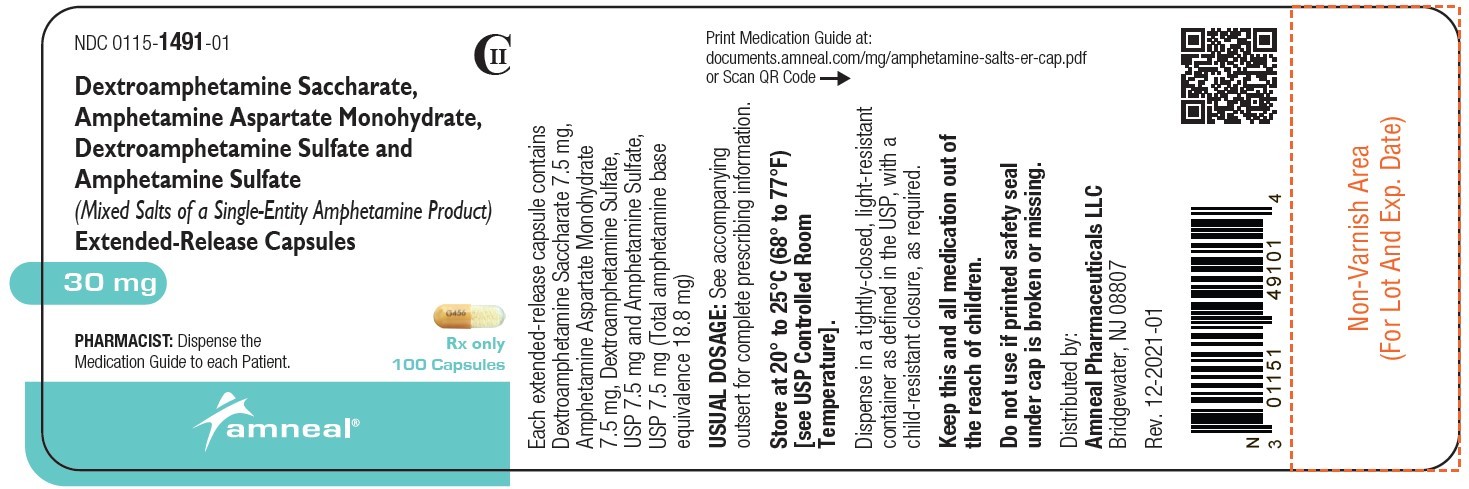
| NDC | 0115-1486-01, 0115-1487-01, 0115-1488-01, 0115-1489-01, 0115-1490-01, 0115-1491-01 |
| Set ID | 89c4b21a-1a65-41ef-8718-1619c2ba8591 |
| Category | HUMAN PRESCRIPTION DRUG LABEL |
| Packager | Amneal Pharmaceuticals of New York LLC |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number | ANDA076852 |