| NDC | 63479-0407-1 |
| Set ID | c287b39d-d0cd-4e5d-8930-6f07f2ed89de |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Apex Energetics Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
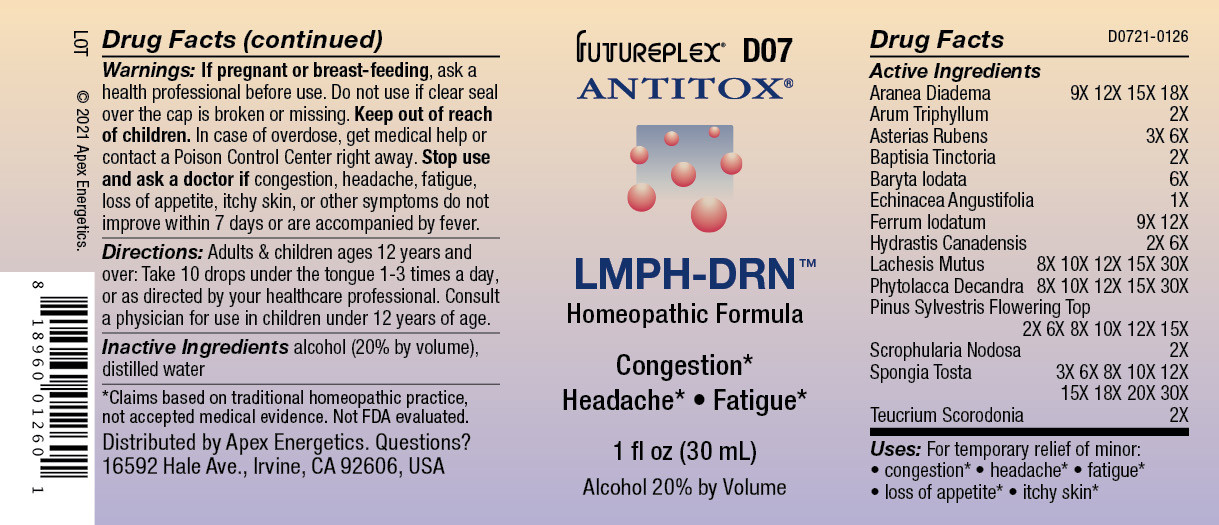
ACTIVE INGREDIENT
Active Ingredients
Aranea Diadema
9X 12X 15X 18X
Arum Triphyllum
2X
Asterias Rubens
3X 6X
Baptisia Tinctoria
2X
Baryta Iodata
6X
Echinacea Angustifolia
1X
Ferrum Iodatum
9X 12X
Hydrastis Canadensis
2X 6X
Lachesis Mutus
8X 10X 12X 15X 30X
Phytolacca Decandra
8X 10X 12X 15X 30X
Pinus Sylvestris Flowering Top
2X 6X 8X 10X 12X 15X
Scrophularia Nodosa
2X
Spongia Tosta
3X 6X 8X 10X 12X 15X 18X 20X 30X
Teucrium Scorodonia
2X
- INDICATIONS & USAGE
- Warnings:
- Directions:
- Inactive Ingredients
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
D07 LMPH-DRN
araneus diadematus, arisaema triphyllum root, asterias rubens, baptisia tinctoria, barium iodide, echinacea angustifolia, ferrous iodide, goldenseal, lachesis muta venom, phytolacca americana root, pinus sylvestris flowering top, scrophularia nodosa, spongia officinalis skeleton, roasted, and teucrium scorodonia flowering top solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63479-0407 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARANEUS DIADEMATUS (UNII: 6T6CO7R3Z5) (ARANEUS DIADEMATUS - UNII:6T6CO7R3Z5) ARANEUS DIADEMATUS 18 [hp_X] in 1 mL ARISAEMA TRIPHYLLUM ROOT (UNII: DM64K844DM) (ARISAEMA TRIPHYLLUM ROOT - UNII:DM64K844DM) ARISAEMA TRIPHYLLUM ROOT 2 [hp_X] in 1 mL ASTERIAS RUBENS (UNII: A7FYY9Q742) (ASTERIAS RUBENS - UNII:A7FYY9Q742) ASTERIAS RUBENS 6 [hp_X] in 1 mL BAPTISIA TINCTORIA ROOT (UNII: 5EF0HWI5WU) (BAPTISIA TINCTORIA ROOT - UNII:5EF0HWI5WU) BAPTISIA TINCTORIA ROOT 2 [hp_X] in 1 mL BARIUM IODIDE (UNII: WKC4T7680A) (BARIUM IODIDE - UNII:WKC4T7680A) BARIUM IODIDE 6 [hp_X] in 1 mL ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 1 [hp_X] in 1 mL FERROUS IODIDE (UNII: F5452U54PN) (FERROUS IODIDE - UNII:F5452U54PN) FERROUS IODIDE 12 [hp_X] in 1 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 6 [hp_X] in 1 mL LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 30 [hp_X] in 1 mL PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 30 [hp_X] in 1 mL PINUS SYLVESTRIS FLOWERING TOP (UNII: 2HEM73YI9I) (PINUS SYLVESTRIS FLOWERING TOP - UNII:2HEM73YI9I) PINUS SYLVESTRIS FLOWERING TOP 15 [hp_X] in 1 mL SCROPHULARIA NODOSA (UNII: 7H443NUB2T) (SCROPHULARIA NODOSA - UNII:7H443NUB2T) SCROPHULARIA NODOSA 2 [hp_X] in 1 mL SPONGIA OFFICINALIS SKELETON, ROASTED (UNII: 1PIP394IID) (SPONGIA OFFICINALIS SKELETON, ROASTED - UNII:1PIP394IID) SPONGIA OFFICINALIS SKELETON, ROASTED 30 [hp_X] in 1 mL TEUCRIUM SCORODONIA FLOWERING TOP (UNII: LOK3I16O7G) (TEUCRIUM SCORODONIA FLOWERING TOP - UNII:LOK3I16O7G) TEUCRIUM SCORODONIA FLOWERING TOP 2 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63479-0407-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 08/01/1988 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/01/1988 Labeler - Apex Energetics Inc. (195816384)