| NDC | 70931-0003-1 |
| Set ID | 3bbcb812-feff-3b00-e054-00144ff88e88 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | DRAN CO.,LTD |
| Generic Name | |
| Product Class | Adenosine Receptor Agonist |
| Product Number | |
| Application Number |
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
1. Do not use in the following cases(Eczema and scalp wounds)
2.Side Effects
1)Due to the use of this product, if rash, irritation, itching and symptopms of hypersnesitivity occur dicontinue use and consult your pharmacist or doctor
3.General Precautions
1)If in contact with the eyes, wash out thoroughty with water If the symptoms are servere, seek medical advice immediately
2)This product is for exeternal use only. Do not use for internal use
4.Storage and handling precautions
1)If possible, avoid direct sunlight and store in cool and area of low humidity
2)In order to maintain the quality of the product and avoid misuse
3)Avoid placing the product near fire and store out in reach of children - DOSAGE & ADMINISTRATION
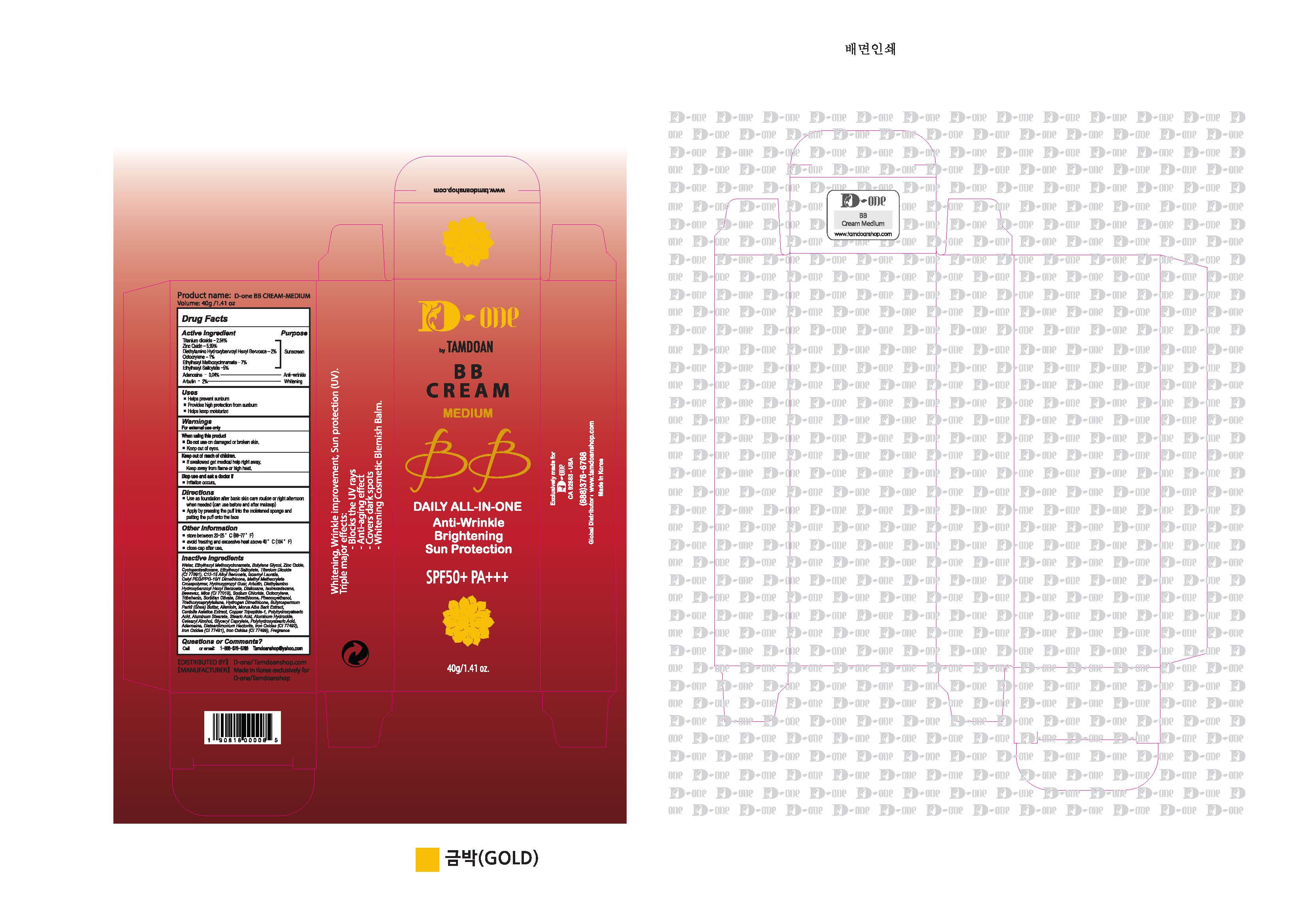
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
D-ONE BY TAMDOAN BB MEDIUM
titanium dioxide, zinc oxide, diethylamino hydroxybenzoyl hexyl benzoate, octocrylene, ethylhexyl methoxycinnamate, ethylhexyl salicylate, adenosine, arbutin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70931-0003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 2.54 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 5.99 g in 100 g DIETHYLAMINO HYDROXYBENZOYL HEXYL BENZOATE (UNII: ANQ870JD20) (DIETHYLAMINO HYDROXYBENZOYL HEXYL BENZOATE - UNII:ANQ870JD20) DIETHYLAMINO HYDROXYBENZOYL HEXYL BENZOATE 2 g in 100 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 1 g in 100 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g ADENOSINE (UNII: K72T3FS567) (ADENOSINE - UNII:K72T3FS567) ADENOSINE 0.04 g in 100 g ARBUTIN (UNII: C5INA23HXF) (ARBUTIN - UNII:C5INA23HXF) ARBUTIN 2 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALLANTOIN (UNII: 344S277G0Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70931-0003-1 40 g in 1 TUBE; Type 0: Not a Combination Product 11/19/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/19/2019 Labeler - DRAN CO.,LTD (689516973) Registrant - DRAN CO.,LTD (689516973) Establishment Name Address ID/FEI Business Operations DRAN CO.,LTD 689516973 manufacture(70931-0003)