| NDC | 63776-073-15 |
| Set ID | f5171818-902d-8ee3-da28-5048a97aa2a6 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | VIATREXX BIO INCORPORATED |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- Active Ingredients
- Purpose:
- Uses
- Warnings
- Dosage
- Other Ingredients
- Other Information
- Questions
-
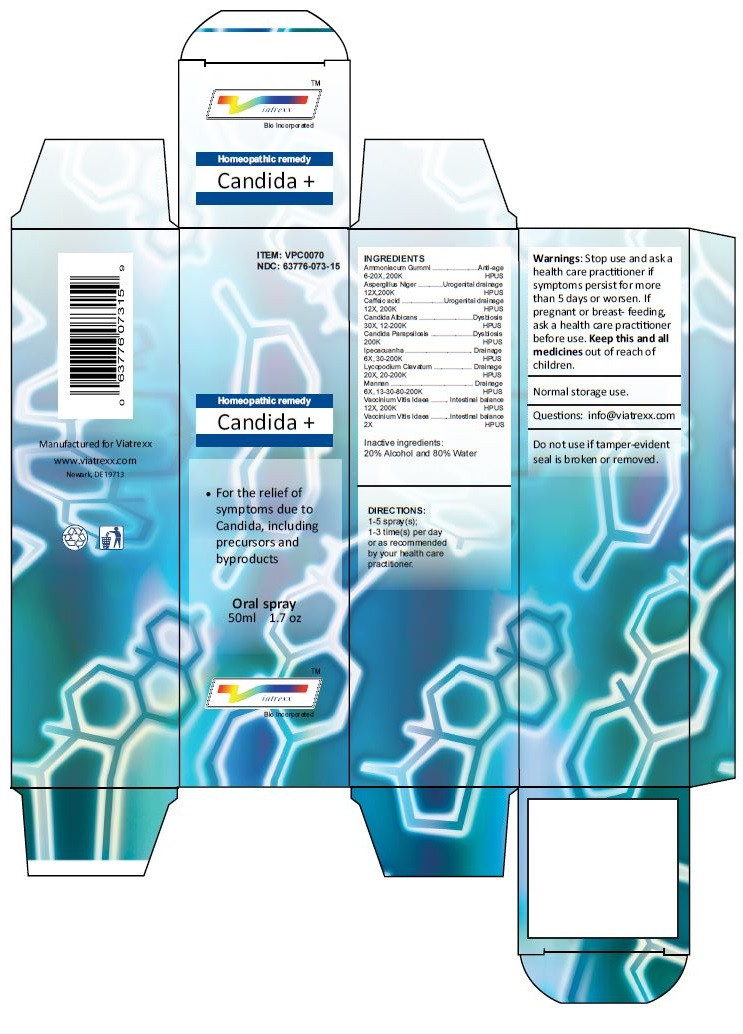
Principal Display Panel
ITEM: VPC0070
NDC 63776-073-15
Homeopathic remedy
Candida +
• For the relief of symptoms due to Candida, including precursors and byproducts
Oral spray
50ml 1.7 oz
Viatrexx™ Bio Incorporated
Manufactured by Viatrexx
www.viatrexx.com
Newark, DE 19713
Candida +
50 mL
1.7 oz
Viatrexx™ Bio Incorporated
ITEM: VPC0070
NDC: 63776-073-15
INDICATIONS:
For the relief of symptoms due to Candida, including precursors and byproducts
DIRECTIONS:
1-5 spray(s); 1-3 time(s) per day or as recommended by your health care practitioner.
Mfg. for
Viatrexx Bio Incorporated.
www.viatrexx.com
Newark, DE 19713
-
INGREDIENTS AND APPEARANCE
CANDIDA
ammoniac, aspergillus niger var. niger, caffeic acid, candida albicans, candida parapsilosis, ipecac, lycopodium clavatum spore, yeast mannan, vaccinium vitis-idaea leaf sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63776-073 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Ammoniac (UNII: 8471AK50P8) (Ammoniac - UNII:8471AK50P8) Ammoniac 200 [kp_C] in 1 mL Aspergillus Niger Var. Niger (UNII: 9IOA40ANG6) (Aspergillus Niger Var. Niger - UNII:9IOA40ANG6) Aspergillus Niger Var. Niger 200 [kp_C] in 1 mL Caffeic Acid (UNII: U2S3A33KVM) (Caffeic Acid - UNII:U2S3A33KVM) Caffeic Acid 200 [kp_C] in 1 mL Candida Albicans (UNII: 4D7G21HDBC) (Candida Albicans - UNII:4D7G21HDBC) Candida Albicans 200 [kp_C] in 1 mL Candida Parapsilosis (UNII: 0KZ676D44N) (Candida Parapsilosis - UNII:0KZ676D44N) Candida Parapsilosis 200 [kp_C] in 1 mL Ipecac (UNII: 62I3C8233L) (Ipecac - UNII:62I3C8233L) Ipecac 200 [kp_C] in 1 mL Lycopodium Clavatum Spore (UNII: C88X29Y479) (Lycopodium Clavatum Spore - UNII:C88X29Y479) Lycopodium Clavatum Spore 200 [kp_C] in 1 mL Yeast Mannan (UNII: 91R887N59P) (Yeast Mannan - UNII:91R887N59P) Yeast Mannan 200 [kp_C] in 1 mL Vaccinium Vitis-idaea Leaf (UNII: FO2ACM0RMQ) (Vaccinium Vitis-idaea Leaf - UNII:FO2ACM0RMQ) Vaccinium Vitis-idaea Leaf 200 [kp_C] in 1 mL Inactive Ingredients Ingredient Name Strength Alcohol (UNII: 3K9958V90M) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63776-073-15 1 in 1 BOX 07/24/2012 1 50 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/24/2012 Labeler - VIATREXX BIO INCORPORATED (078419880) Establishment Name Address ID/FEI Business Operations Les Importations Herbasante Inc 243254612 MANUFACTURE(63776-073)