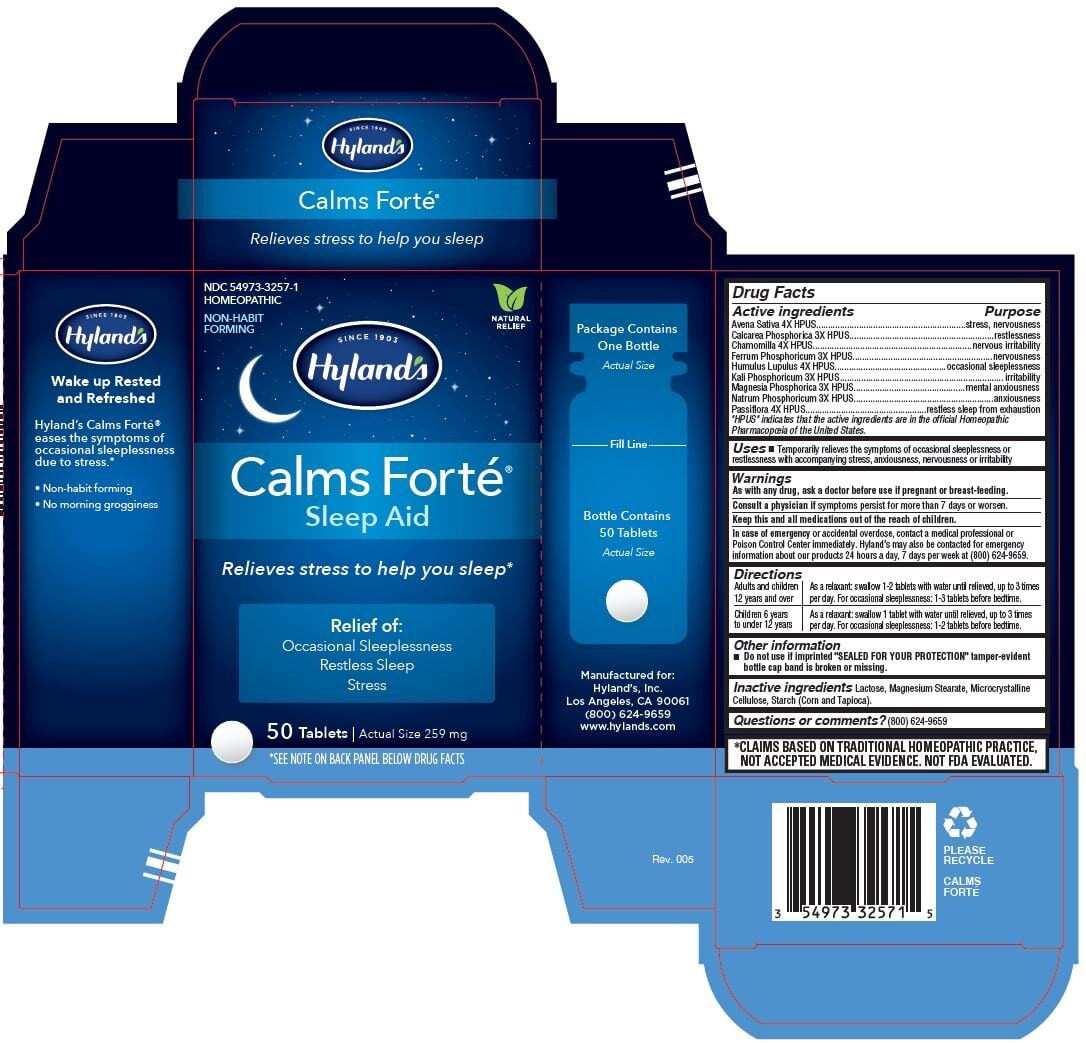
| NDC | 54973-3257-1, 54973-3257-2, 54973-3257-3 |
| Set ID | 63ce9942-a299-4d9b-b63b-35b297472dc9 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Hyland's |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- SPL UNCLASSIFIED SECTION
-
ACTIVE INGREDIENT
Active ingredients Purpose "HPUS" indicates the active ingredients are in the official Homeopathic Pharmacopœia of the United States. Avena Sativa 4X HPUS stress, nervousness Calcarea Phosphorica 3X HPUS restlessness Chamomilla 4X HPUS nervous irritability Ferrum Phosphoricum 3X HPUS nervousness Humulus Lupulus 4X HPUS occasional sleeplessness Kali Phosphoricum 3X HPUS irritability Magnesia Phosphorica 3X HPUS mental anxiousness Natrum Phosphoricum 3X HPUS anxiousness Passiflora 4X HPUS restless sleep from exhaustion - Uses
- Warnings
-
Directions
Adults and children
12 years and over
As a relaxant: swallow 1-2 tablets with water until relieved, up to 3 times
per day. For occasional sleeplessness: 1-3 tablets before bedtime.
Children 6 years
to under 12 years
As a relaxant: swallow 1 tablet with water until relieved, up to 3 times
per day. For occasional sleeplessness: 1-2 tablets before bedtime.
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 Tablet Bottle Carton
-
INGREDIENTS AND APPEARANCE
CALMS FORTE
avena sativa flowering top, tribasic calcium phosphate, matricaria recutita, ferrosoferric phosphate, hops, potassium phosphate, dibasic, magnesium phosphate, dibasic trihydrate, sodium phosphate, dibasic, heptahydrate, and passiflora incarnata flower tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54973-3257 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVENA SATIVA FLOWERING TOP (UNII: MA9CQJ3F7F) (AVENA SATIVA FLOWERING TOP - UNII:MA9CQJ3F7F) AVENA SATIVA FLOWERING TOP 4 [hp_X] TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) (PHOSPHATE ION - UNII:NK08V8K8HR) TRIBASIC CALCIUM PHOSPHATE 3 [hp_X] MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 4 [hp_X] FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERROSOFERRIC PHOSPHATE 3 [hp_X] HOPS (UNII: 01G73H6H83) (HOPS - UNII:01G73H6H83) HOPS 4 [hp_X] POTASSIUM PHOSPHATE, DIBASIC (UNII: CI71S98N1Z) (PHOSPHATE ION - UNII:NK08V8K8HR) POTASSIUM PHOSPHATE, DIBASIC 3 [hp_X] MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE (UNII: HF539G9L3Q) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE 3 [hp_X] SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE 3 [hp_X] PASSIFLORA INCARNATA FLOWER (UNII: K8F3G29S6Z) (PASSIFLORA INCARNATA FLOWER - UNII:K8F3G29S6Z) PASSIFLORA INCARNATA FLOWER 4 [hp_X] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) MAGNESIUM PALMITOSTEARATE (UNII: R4OXA9G5BV) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) STARCH, TAPIOCA (UNII: 24SC3U704I) Product Characteristics Color white Score no score Shape ROUND Size 9mm Flavor Imprint Code HYL;CF Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54973-3257-1 1 in 1 CARTON 04/29/2016 1 50 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:54973-3257-2 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/29/2016 3 NDC:54973-3257-3 4 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/29/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/29/2016 Labeler - Hyland's (028570695) Establishment Name Address ID/FEI Business Operations Standard Homeopathic Company 008316655 manufacture(54973-3257) , pack(54973-3257)