| NDC | 43772-0037-1 |
| Set ID | cdf4b153-3c92-4e3e-b3ac-f41153fc3941 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Synergy Formulas, Inc |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
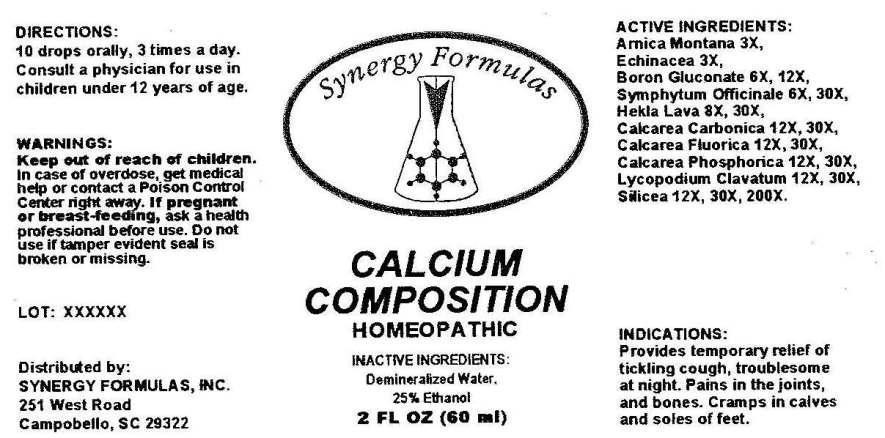
- ACTIVE INGREDIENTS:
- INDICATIONS:
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN.
- DIRECTIONS:
- INDICATIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
CALCIUM COMPOSITION
arnica montana, echinacea (angustifolia), boron glucconate, symphytum officinale, hekla lava, calcarea carbonica, clacarea fluorica, calcarea phosphorica, lycopodium clavatum, silicea. liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43772-0037 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 3 [hp_X] in 1 mL ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 3 [hp_X] in 1 mL BORON GLUCONATE (UNII: X8P2SY4Y7V) (BORON - UNII:N9E3X5056Q) BORON GLUCONATE 6 [hp_X] in 1 mL COMFREY ROOT (UNII: M9VVZ08EKQ) (COMFREY ROOT - UNII:M9VVZ08EKQ) COMFREY ROOT 6 [hp_X] in 1 mL HEKLA LAVA (UNII: C21158IIRK) (HEKLA LAVA - UNII:C21158IIRK) HEKLA LAVA 8 [hp_X] in 1 mL OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 12 [hp_X] in 1 mL CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 12 [hp_X] in 1 mL TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 12 [hp_X] in 1 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 12 [hp_X] in 1 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (COLLOIDAL SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43772-0037-1 60 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/16/2014 Labeler - Synergy Formulas, Inc (069579220) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(43772-0037) , api manufacture(43772-0037) , label(43772-0037) , pack(43772-0037)