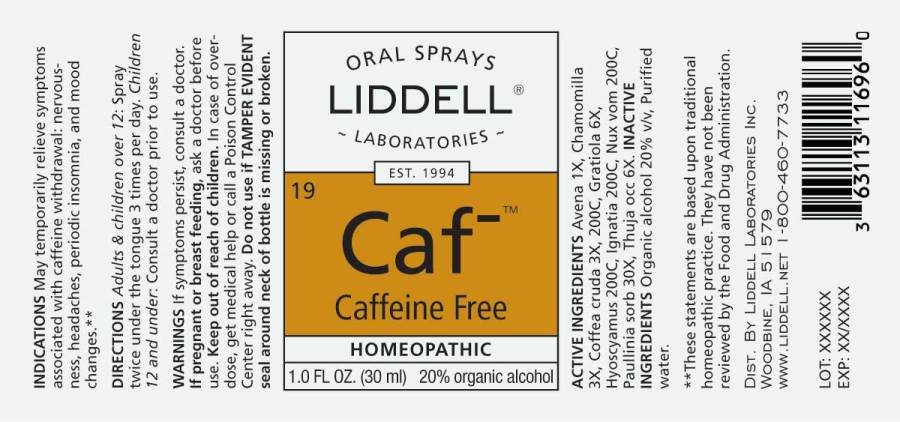
| NDC | 50845-0155-1 |
| Set ID | 61c7e82e-eb4d-43ac-aacb-2a9ae23bd51e |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Liddell Laboratories, Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- ACTIVE INGREDIENTS:
- INDICATIONS:
- WARNINGS:
- DIRECTIONS:
- INACTIVE INGREDIENTS:
- KEEP OUT OF REACH OF CHILDREN:
- INDICATIONS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
CAFFEINE FREE
avena sativa, chamomilla, coffea cruda, gratiola officinalis, hyoscyamus niger, ignatia amara, nux vomica, paullinia sorbilis, thuja occidentalis, sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50845-0155 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVENA SATIVA FLOWERING TOP (UNII: MA9CQJ3F7F) (AVENA SATIVA FLOWERING TOP - UNII:MA9CQJ3F7F) AVENA SATIVA FLOWERING TOP 1 [hp_X] in 1 mL MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 3 [hp_X] in 1 mL ARABICA COFFEE BEAN (UNII: 3SW678MX72) (ARABICA COFFEE BEAN - UNII:3SW678MX72) ARABICA COFFEE BEAN 3 [hp_X] in 1 mL GRATIOLA OFFICINALIS (UNII: BDD9991A36) (GRATIOLA OFFICINALIS - UNII:BDD9991A36) GRATIOLA OFFICINALIS 6 [hp_X] in 1 mL HYOSCYAMUS NIGER (UNII: 4WRK2153H3) (HYOSCYAMUS NIGER - UNII:4WRK2153H3) HYOSCYAMUS NIGER 200 [hp_C] in 1 mL STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 200 [hp_C] in 1 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 200 [hp_C] in 1 mL PAULLINIA CUPANA SEED (UNII: C21GE7524R) (PAULLINIA CUPANA SEED - UNII:C21GE7524R) PAULLINIA CUPANA SEED 30 [hp_X] in 1 mL THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 6 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50845-0155-1 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 05/24/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/16/2011 Labeler - Liddell Laboratories, Inc. (832264241) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(50845-0155) , api manufacture(50845-0155) , label(50845-0155) , pack(50845-0155)