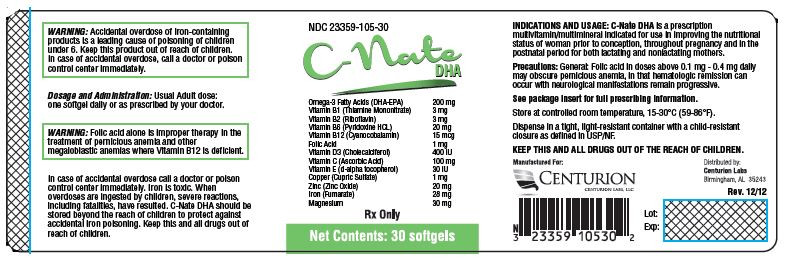
| NDC | 23359-105-01, 23359-105-05, 23359-105-30 |
| Set ID | 8c1b2367-cec5-4a18-82a0-e132b4aece3a |
| Category | HUMAN PRESCRIPTION DRUG LABEL |
| Packager | Centurion Labs, LLC |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- SPL UNCLASSIFIED SECTION
- Description:
- DESCRIPTION
Close
Each Softgel Contains: Omega-3 Fatty Acids (DHA-EPA) 200mg Vitamin B1 (Thiamine Mononitrate) 3 mg Vitamin B2 (Riboflavin) 3 mg Vitamin B6 (Pyridoxine HCL) 20 mg Vitamin B12 (Cyanocobalamin) 15 mcg Folic Acid 1 mg Vitamin D3 (Cholecalciferol) 400 IU Vitamin C (Ascorbic Acid) 100 mg Vitamin E (d-alpha tocopherol) 30 IU Copper (Cupric Sulfate) 1 mg Zinc (Zinc Oxide) 20 mg Iron (Fumerate) 28 mg Magnesium 30 mg - INACTIVE INGREDIENT
INACTIVE INGREDIENTS: Gelatin, Glycerol, beeswax yellow, lecithin, water-purified and colorant.
Close - INDICATIONS & USAGE
INDICATIONS AND USAGE: C-NATE DHA is a prescription multivitamin/multimineral indicated for use in improving the nutritional status of women prior to conception, throughout pregnancy and in the postnatal period for both lactating and nonlactating mothers.
Close - CONTRAINDICATIONS
CONTRAINDICATIONS: C-NATE DHA should not be used by patients with known history of hypersensitivity to any of the listed ingredients.
Close - PRECAUTIONS
PRECAUTIONS: Folic Acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic Acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress. Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
Close - WARNINGS
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately.
Close - PEDIATRIC USE
- GERIATRIC USE
GERIACTRIC USE: No clinical studies have been performed in patients over 65 to determine whether older persons respond differently from younger persons. Physicians should consider that elderly person may have decreased hepatic, renal, or cardiac function.
Close - DRUG INTERACTIONS
DRUG INTERACTIONS: C-NATE DHA softgels are not recommended for and should not be given to patients receiving levodopa because the action of levodopa is antagonized by pyridoxine.
Close - ADVERSE REACTIONS
ADVERSE REACTIONS: Adverse reactions have been reported with specific vitamins and minerals but generally at levels substantially higher than those contained herein. However, allergic and idiosyncratic reactions are possible at lower levels. Iron, even at the usual recommended levels, has been associated with gastrointestinal intolerance in some patients.
Close - DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION: Usual adult dose: one softgel daily or as prescribed by your doctor.
Close - HOW SUPPLIED
HOW SUPPLIED: C-NATE DHA is available as a Annato colored softgel, imprinted PRE 01. Available in 30 count bottles with NDC #23359-105-30 and samples with NDC #23359-105-05 and NDC #23359-105-01.
PHYSICIAN SAMPLE NOT FOR RESALE
Close - STORAGE AND HANDLING
Recommended Storage: 15°-30°C (59°-86°F) degrees. Protect from light moisture and avoid excessive heat. Dispense original carton.
KEEP OUT OF REACH OF CHILDREN
RX ONLY
Close - SPL UNCLASSIFIED SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 23359-105-30
C-Nate
DHASoftgels Omega-3 Fatty Acids (DHA-EPA) 200mg Vitamin B1 (Thiamine Mononitrate) 3 mg Vitamin B2 (Riboflavin) 3 mg Vitamin B6 (Pyridoxine HCL) 20 mg Vitamin B12 (Cyanocobalamin) 15 mcg Folic Acid 1 mg Vitamin D3 (Cholecalciferol) 400 IU Vitamin C (Ascorbic Acid) 100 mg Vitamin E (d-alpha tocopherol) 30 IU Copper (Cupric Sulfate) 1 mg Zinc (Zinc Oxide) 20 mg Iron (Fumerate) 28 mg Magnesium 30 mg Rx Only
Net Contents: 30 softgels
Manufactured For:
Centurion Labs LLCDistributed by:
Centurion Labs LLC
Birmingham, AL 35243Close
- INGREDIENTS AND APPEARANCE
C-NATE DHA
omega-3 fatty acids, icosapent, doconexent, thiamine mononitrate, riboflavin, pyridoxine hydrochloride, cyanocobalamin, folic acid, cholecalciferol, ascorbic acid, .alpha.-tocopherol, d-, cupric sulfate, zinc oxide, ferrous fumarate and magnesium oxide capsule, gelatin coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:23359-105 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OMEGA-3 FATTY ACIDS (UNII: 71M78END5S) (OMEGA-3 FATTY ACIDS - UNII:71M78END5S) OMEGA-3 FATTY ACIDS 200 mg THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 3 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 3 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 20 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 15 ug FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 400 [iU] ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 100 mg .ALPHA.-TOCOPHEROL, D- (UNII: N9PR3490H9) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL, D- 30 [iU] CUPRIC SULFATE (UNII: LRX7AJ16DT) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 1 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 20 mg FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 28 mg MAGNESIUM (UNII: I38ZP9992A) (MAGNESIUM - UNII:I38ZP9992A) MAGNESIUM 30 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) YELLOW WAX (UNII: 2ZA36H0S2V) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) WATER (UNII: 059QF0KO0R) Product Characteristics Color BROWN (annato) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code PRE;01 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23359-105-30 30 in 1 BOTTLE 2 NDC:23359-105-05 5 in 1 CARTON 3 NDC:23359-105-01 1 in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/2013 CloseLabeler - Centurion Labs, LLC (806756461)