| NDC | 98132-710-01, 98132-711-01, 98132-712-01, 98132-713-01, 98132-714-01, 98132-715-01, 98132-716-01, 98132-717-01, 98132-718-01, 98132-719-01, 98132-762-01, 98132-763-01 |
| Set ID | 5a451bf6-3383-4955-a4db-2e41573d26c9 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Bare Escentuals Beauty, Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number | PART352 |
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months: ask a doctor
- Sun protection measures. Spending time in the sun increases your risk of skin cancer and early skin aging.
To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
-
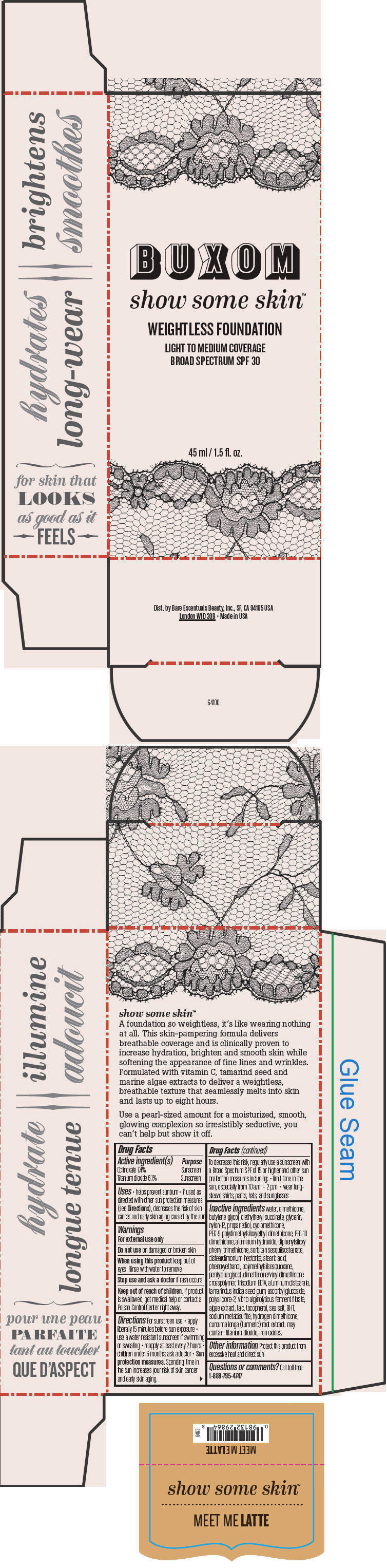
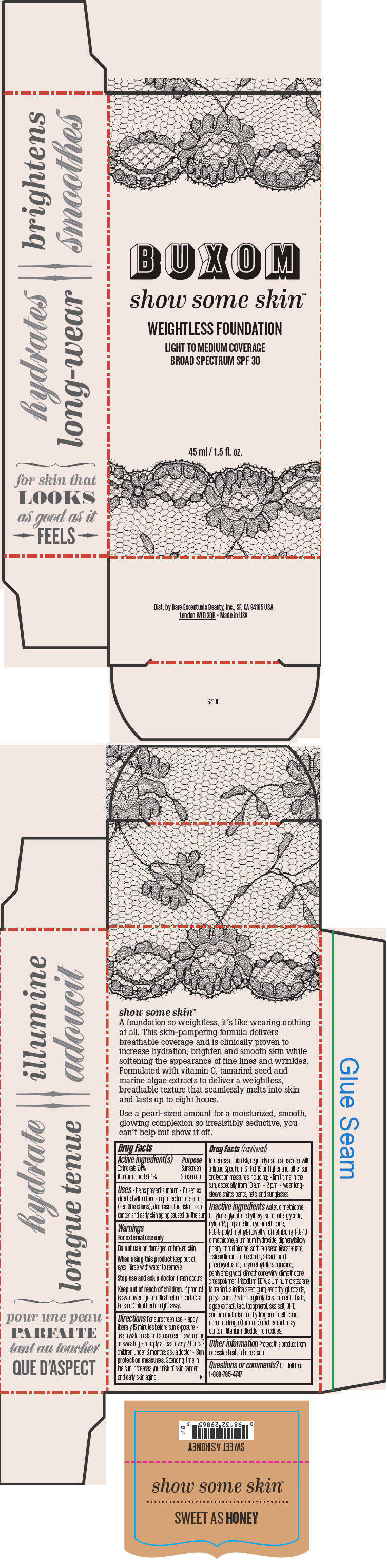
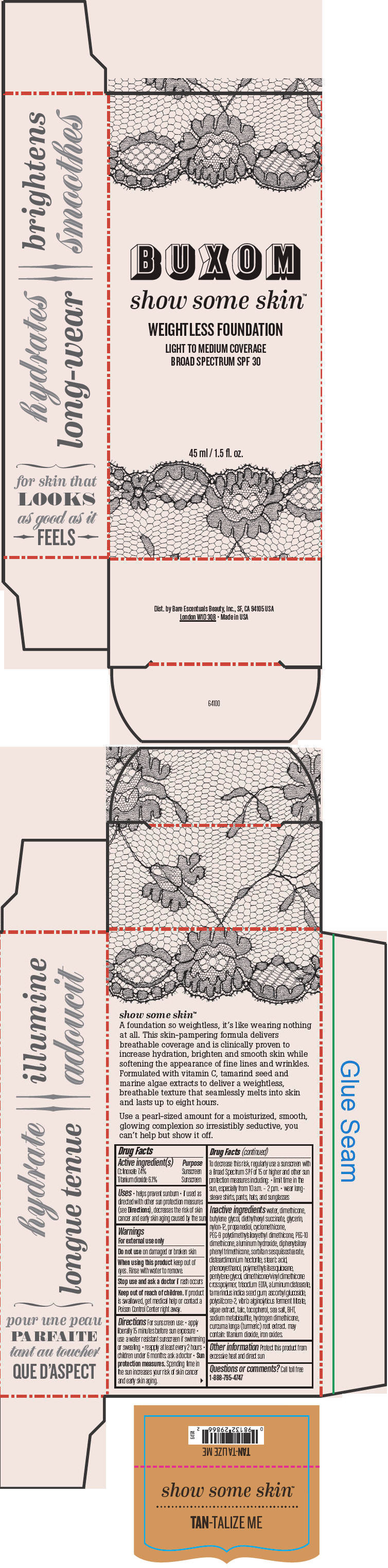
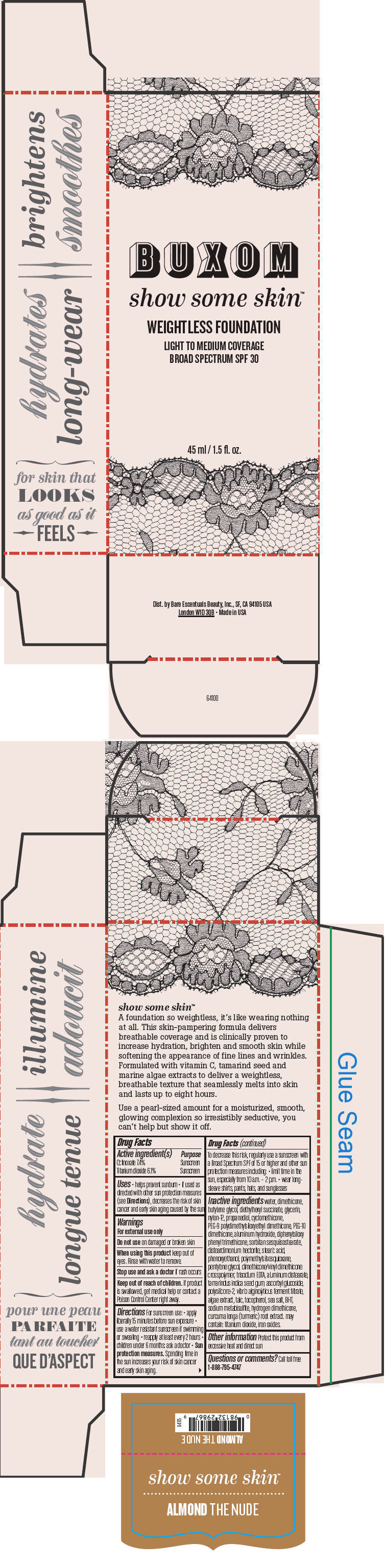
Inactive ingredients
water, dimethicone, butylene glycol, diethylhexyl succinate, glycerin, nylon-12, propanediol, cyclomethicone, PEG-9 polydimethylsiloxyethyl dimethicone, PEG-10 dimethicone, aluminum hydroxide, diphenylsiloxy phenyl trimethicone, sorbitan sesquiisostearate, disteardimonium hectorite, stearic acid, phenoxyethanol, polymethylsilsesquioxane, pentylene glycol, dimethicone/vinyl dimethicone crosspolymer, trisodium EDTA, aluminum distearate, tamarindus indica seed gum, ascorbyl glucoside, polysilicone-2, vibrio alginolyticus ferment filtrate, algae extract, talc, tocopherol, sea salt, BHT, sodium metabisulfite, hydrogen dimethicone, curcuma longa (turmeric) root extract. may contain: titanium dioxide, iron oxides.
- Other information
- Questions or comments?
- SPL UNCLASSIFIED SECTION
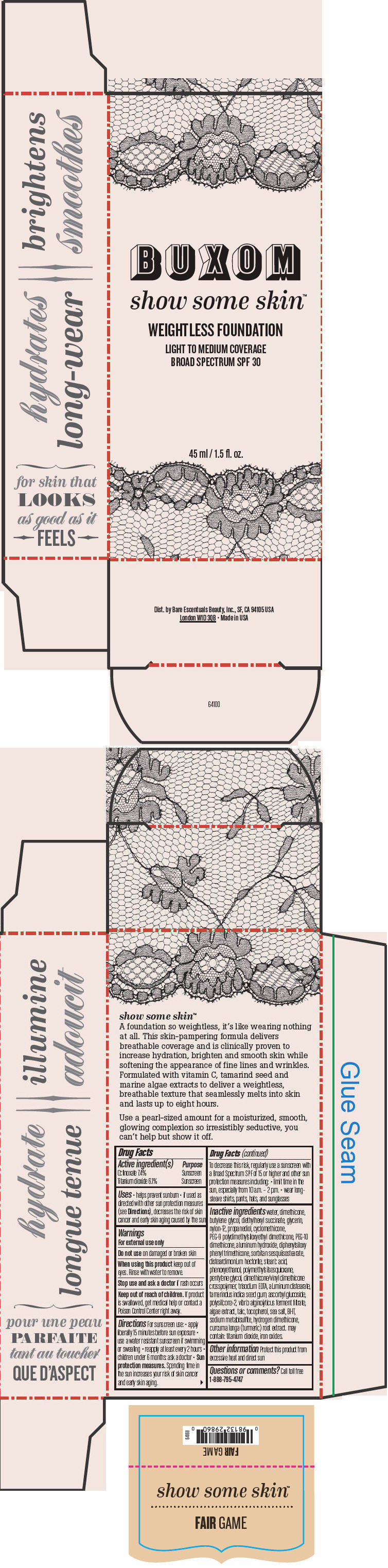
- PRINCIPAL DISPLAY PANEL - 45 ml Tube Box - Fair Game
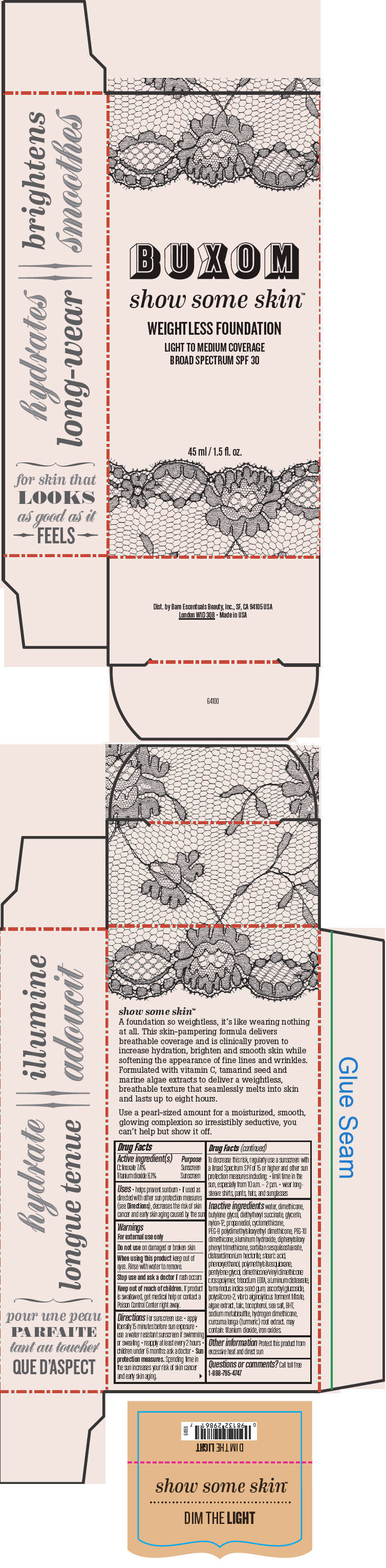
- PRINCIPAL DISPLAY PANEL - 45 ml Tube Box - Dim The Light
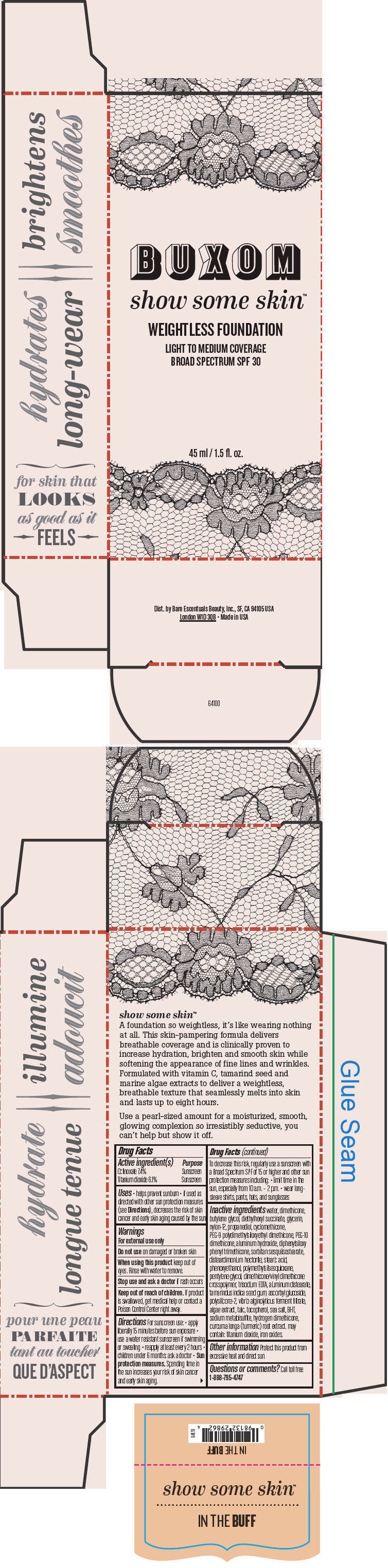
- PRINCIPAL DISPLAY PANEL - 45 ml Tube Box - In The Buff
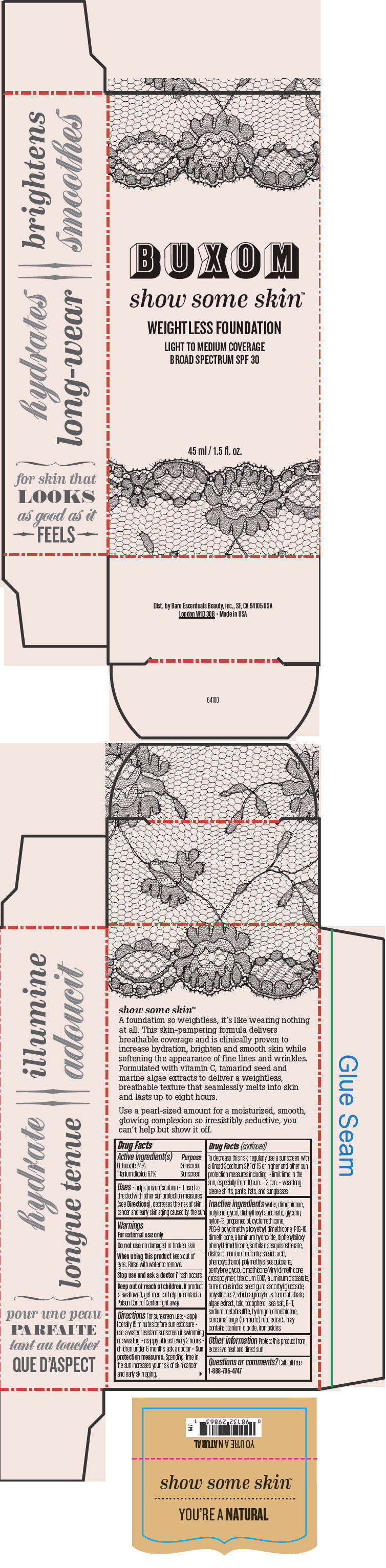
- PRINCIPAL DISPLAY PANEL - 45 ml Tube Box - You're A Natural
- PRINCIPAL DISPLAY PANEL - 45 ml Tube Box - Meet Me Latte
- PRINCIPAL DISPLAY PANEL - 45 ml Tube Box - Sweet As Honey
- PRINCIPAL DISPLAY PANEL - 45 ml Tube Box - Tan-Talize Me
- PRINCIPAL DISPLAY PANEL - 45 ml Tube Box - Almond The Nude
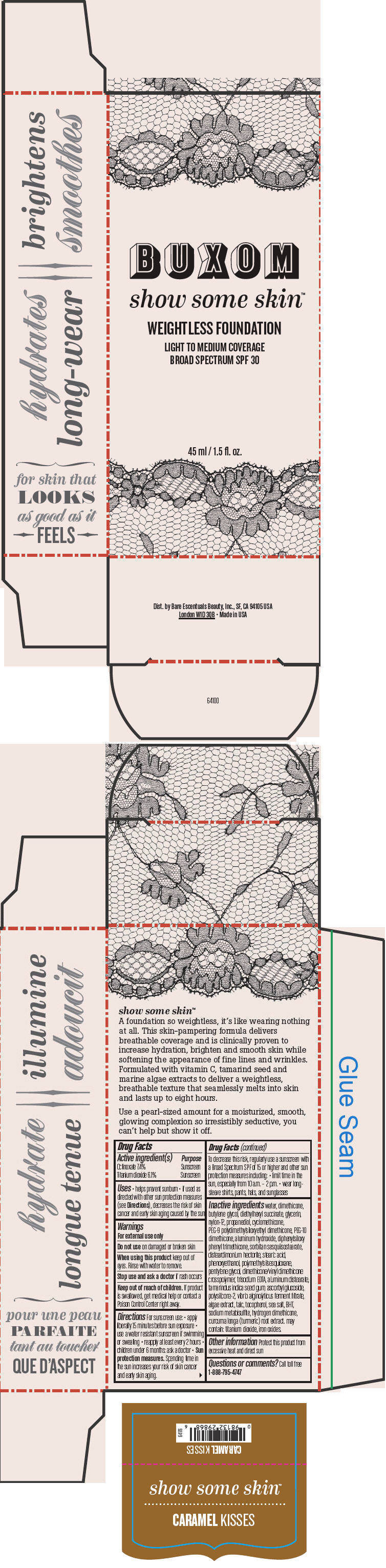
- PRINCIPAL DISPLAY PANEL - 45 ml Tube Box - Caramel Kisses
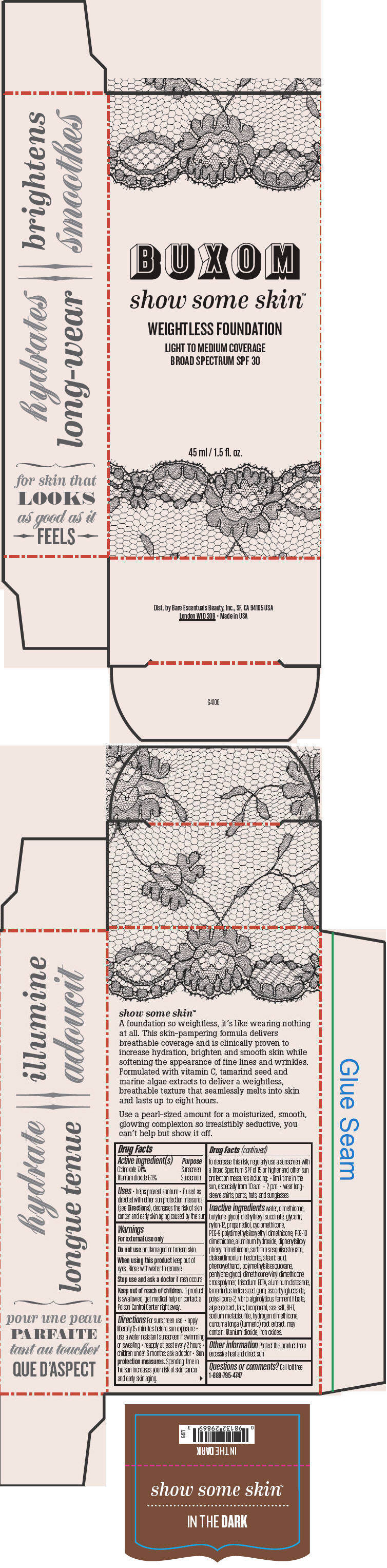
- PRINCIPAL DISPLAY PANEL - 45 ml Tube Box - In The Dark
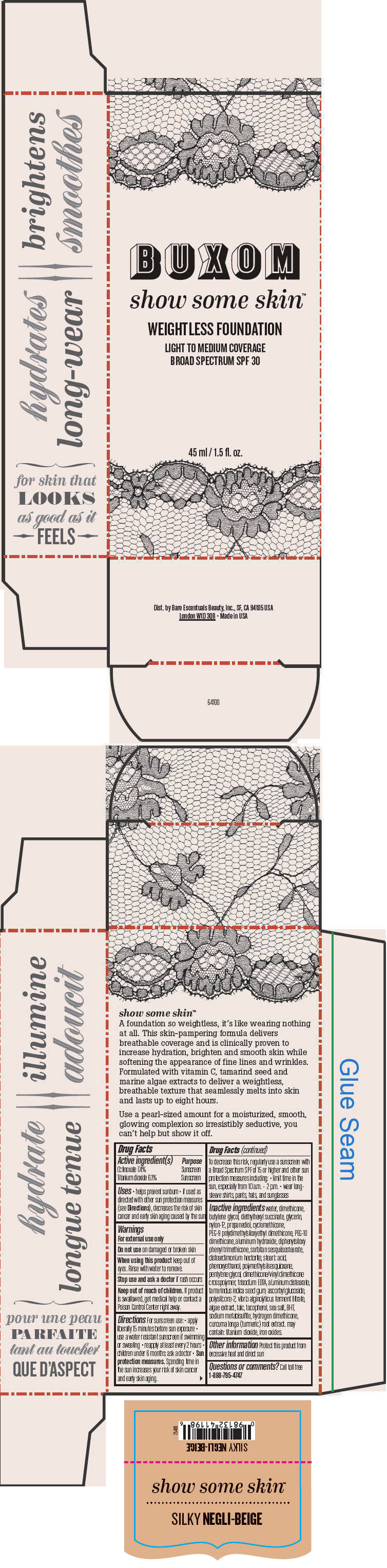
- PRINCIPAL DISPLAY PANEL - 45 ml Tube Box - Silky Negli-Beige
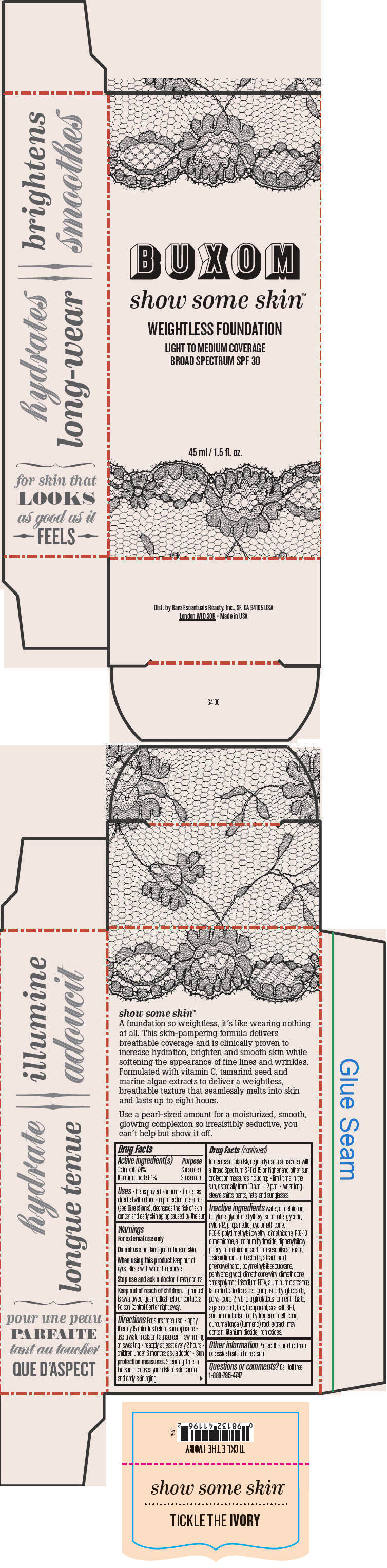
- PRINCIPAL DISPLAY PANEL - 45 ml Tube Box - Tickle The Ivory
-
INGREDIENTS AND APPEARANCE
BUXOM SHOW SOME SKIN WEIGHTLESS FOUNDATION BROAD SPECTRUM SPF 30 FAIR GAME
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-710 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 74 mg in 1 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 61 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) dimethicone (UNII: 92RU3N3Y1O) butylene glycol (UNII: 3XUS85K0RA) diethylhexyl succinate (UNII: 69W9UMG3P8) glycerin (UNII: PDC6A3C0OX) nylon-12 (UNII: 446U8J075B) propanediol (UNII: 5965N8W85T) cyclomethicone (UNII: NMQ347994Z) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) aluminum hydroxide (UNII: 5QB0T2IUN0) diphenylsiloxy phenyl trimethicone (UNII: I445L28B12) disteardimonium hectorite (UNII: X687XDK09L) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) pentylene glycol (UNII: 50C1307PZG) edetate trisodium (UNII: 420IP921MB) aluminum distearate (UNII: 7P1HP1B9UI) tamarind seed (UNII: 6AHP8A7OML) ascorbyl glucoside (UNII: 2V52R0NHXW) talc (UNII: 7SEV7J4R1U) tocopherol (UNII: R0ZB2556P8) sea salt (UNII: 87GE52P74G) butylated hydroxytoluene (UNII: 1P9D0Z171K) sodium metabisulfite (UNII: 4VON5FNS3C) turmeric (UNII: 856YO1Z64F) ferric oxide red (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-710-01 1 in 1 BOX 1 45 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/30/2014 BUXOM SHOW SOME SKIN WEIGHTLESS FOUNDATION BROAD SPECTRUM SPF 30 DIM THE LIGHT
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-711 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 74 mg in 1 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 61 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) dimethicone (UNII: 92RU3N3Y1O) butylene glycol (UNII: 3XUS85K0RA) diethylhexyl succinate (UNII: 69W9UMG3P8) glycerin (UNII: PDC6A3C0OX) nylon-12 (UNII: 446U8J075B) propanediol (UNII: 5965N8W85T) cyclomethicone (UNII: NMQ347994Z) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) aluminum hydroxide (UNII: 5QB0T2IUN0) diphenylsiloxy phenyl trimethicone (UNII: I445L28B12) disteardimonium hectorite (UNII: X687XDK09L) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) pentylene glycol (UNII: 50C1307PZG) edetate trisodium (UNII: 420IP921MB) aluminum distearate (UNII: 7P1HP1B9UI) tamarind seed (UNII: 6AHP8A7OML) ascorbyl glucoside (UNII: 2V52R0NHXW) talc (UNII: 7SEV7J4R1U) tocopherol (UNII: R0ZB2556P8) sea salt (UNII: 87GE52P74G) butylated hydroxytoluene (UNII: 1P9D0Z171K) sodium metabisulfite (UNII: 4VON5FNS3C) turmeric (UNII: 856YO1Z64F) ferric oxide red (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-711-01 1 in 1 BOX 1 45 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/30/2014 BUXOM SHOW SOME SKIN WEIGHTLESS FOUNDATION BROAD SPECTRUM SPF 30 IN THE BUFF
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-712 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 74 mg in 1 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 61 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) dimethicone (UNII: 92RU3N3Y1O) butylene glycol (UNII: 3XUS85K0RA) diethylhexyl succinate (UNII: 69W9UMG3P8) glycerin (UNII: PDC6A3C0OX) nylon-12 (UNII: 446U8J075B) propanediol (UNII: 5965N8W85T) cyclomethicone (UNII: NMQ347994Z) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) aluminum hydroxide (UNII: 5QB0T2IUN0) diphenylsiloxy phenyl trimethicone (UNII: I445L28B12) disteardimonium hectorite (UNII: X687XDK09L) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) pentylene glycol (UNII: 50C1307PZG) edetate trisodium (UNII: 420IP921MB) aluminum distearate (UNII: 7P1HP1B9UI) tamarind seed (UNII: 6AHP8A7OML) ascorbyl glucoside (UNII: 2V52R0NHXW) talc (UNII: 7SEV7J4R1U) tocopherol (UNII: R0ZB2556P8) sea salt (UNII: 87GE52P74G) butylated hydroxytoluene (UNII: 1P9D0Z171K) sodium metabisulfite (UNII: 4VON5FNS3C) turmeric (UNII: 856YO1Z64F) ferric oxide red (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-712-01 1 in 1 BOX 1 45 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/30/2014 BUXOM SHOW SOME SKIN WEIGHTLESS FOUNDATION BROAD SPECTRUM SPF 30 YOURE A NATURAL
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-713 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 74 mg in 1 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 61 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) dimethicone (UNII: 92RU3N3Y1O) butylene glycol (UNII: 3XUS85K0RA) diethylhexyl succinate (UNII: 69W9UMG3P8) glycerin (UNII: PDC6A3C0OX) nylon-12 (UNII: 446U8J075B) propanediol (UNII: 5965N8W85T) cyclomethicone (UNII: NMQ347994Z) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) aluminum hydroxide (UNII: 5QB0T2IUN0) diphenylsiloxy phenyl trimethicone (UNII: I445L28B12) disteardimonium hectorite (UNII: X687XDK09L) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) pentylene glycol (UNII: 50C1307PZG) edetate trisodium (UNII: 420IP921MB) aluminum distearate (UNII: 7P1HP1B9UI) tamarind seed (UNII: 6AHP8A7OML) ascorbyl glucoside (UNII: 2V52R0NHXW) talc (UNII: 7SEV7J4R1U) tocopherol (UNII: R0ZB2556P8) sea salt (UNII: 87GE52P74G) butylated hydroxytoluene (UNII: 1P9D0Z171K) sodium metabisulfite (UNII: 4VON5FNS3C) turmeric (UNII: 856YO1Z64F) ferric oxide red (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-713-01 1 in 1 BOX 1 45 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/30/2014 BUXOM SHOW SOME SKIN WEIGHTLESS FOUNDATION BROAD SPECTRUM SPF 30 MEET ME LATTE
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-714 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 74 mg in 1 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 61 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) dimethicone (UNII: 92RU3N3Y1O) butylene glycol (UNII: 3XUS85K0RA) diethylhexyl succinate (UNII: 69W9UMG3P8) glycerin (UNII: PDC6A3C0OX) nylon-12 (UNII: 446U8J075B) propanediol (UNII: 5965N8W85T) cyclomethicone (UNII: NMQ347994Z) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) aluminum hydroxide (UNII: 5QB0T2IUN0) diphenylsiloxy phenyl trimethicone (UNII: I445L28B12) disteardimonium hectorite (UNII: X687XDK09L) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) pentylene glycol (UNII: 50C1307PZG) edetate trisodium (UNII: 420IP921MB) aluminum distearate (UNII: 7P1HP1B9UI) tamarind seed (UNII: 6AHP8A7OML) ascorbyl glucoside (UNII: 2V52R0NHXW) talc (UNII: 7SEV7J4R1U) tocopherol (UNII: R0ZB2556P8) sea salt (UNII: 87GE52P74G) butylated hydroxytoluene (UNII: 1P9D0Z171K) sodium metabisulfite (UNII: 4VON5FNS3C) turmeric (UNII: 856YO1Z64F) ferric oxide red (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-714-01 1 in 1 BOX 1 45 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/30/2014 BUXOM SHOW SOME SKIN WEIGHTLESS FOUNDATION BROAD SPECTRUM SPF 30 SWEET AS HONEY
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-715 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 74 mg in 1 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 61 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) dimethicone (UNII: 92RU3N3Y1O) butylene glycol (UNII: 3XUS85K0RA) diethylhexyl succinate (UNII: 69W9UMG3P8) glycerin (UNII: PDC6A3C0OX) nylon-12 (UNII: 446U8J075B) propanediol (UNII: 5965N8W85T) cyclomethicone (UNII: NMQ347994Z) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) aluminum hydroxide (UNII: 5QB0T2IUN0) diphenylsiloxy phenyl trimethicone (UNII: I445L28B12) disteardimonium hectorite (UNII: X687XDK09L) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) pentylene glycol (UNII: 50C1307PZG) edetate trisodium (UNII: 420IP921MB) aluminum distearate (UNII: 7P1HP1B9UI) tamarind seed (UNII: 6AHP8A7OML) ascorbyl glucoside (UNII: 2V52R0NHXW) talc (UNII: 7SEV7J4R1U) tocopherol (UNII: R0ZB2556P8) sea salt (UNII: 87GE52P74G) butylated hydroxytoluene (UNII: 1P9D0Z171K) sodium metabisulfite (UNII: 4VON5FNS3C) turmeric (UNII: 856YO1Z64F) ferric oxide red (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-715-01 1 in 1 BOX 1 45 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/30/2014 BUXOM SHOW SOME SKIN WEIGHTLESS FOUNDATION BROAD SPECTRUM SPF 30 TAN-TALIZE ME
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-716 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 74 mg in 1 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 61 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) dimethicone (UNII: 92RU3N3Y1O) butylene glycol (UNII: 3XUS85K0RA) diethylhexyl succinate (UNII: 69W9UMG3P8) glycerin (UNII: PDC6A3C0OX) nylon-12 (UNII: 446U8J075B) propanediol (UNII: 5965N8W85T) cyclomethicone (UNII: NMQ347994Z) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) aluminum hydroxide (UNII: 5QB0T2IUN0) diphenylsiloxy phenyl trimethicone (UNII: I445L28B12) disteardimonium hectorite (UNII: X687XDK09L) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) pentylene glycol (UNII: 50C1307PZG) edetate trisodium (UNII: 420IP921MB) aluminum distearate (UNII: 7P1HP1B9UI) tamarind seed (UNII: 6AHP8A7OML) ascorbyl glucoside (UNII: 2V52R0NHXW) talc (UNII: 7SEV7J4R1U) tocopherol (UNII: R0ZB2556P8) sea salt (UNII: 87GE52P74G) butylated hydroxytoluene (UNII: 1P9D0Z171K) sodium metabisulfite (UNII: 4VON5FNS3C) turmeric (UNII: 856YO1Z64F) ferric oxide red (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-716-01 1 in 1 BOX 1 45 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/30/2014 BUXOM SHOW SOME SKIN WEIGHTLESS FOUNDATION BROAD SPECTRUM SPF 30 ALMOND THE NUDE
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-717 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 74 mg in 1 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 61 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) dimethicone (UNII: 92RU3N3Y1O) butylene glycol (UNII: 3XUS85K0RA) diethylhexyl succinate (UNII: 69W9UMG3P8) glycerin (UNII: PDC6A3C0OX) nylon-12 (UNII: 446U8J075B) propanediol (UNII: 5965N8W85T) cyclomethicone (UNII: NMQ347994Z) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) aluminum hydroxide (UNII: 5QB0T2IUN0) diphenylsiloxy phenyl trimethicone (UNII: I445L28B12) disteardimonium hectorite (UNII: X687XDK09L) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) pentylene glycol (UNII: 50C1307PZG) edetate trisodium (UNII: 420IP921MB) aluminum distearate (UNII: 7P1HP1B9UI) tamarind seed (UNII: 6AHP8A7OML) ascorbyl glucoside (UNII: 2V52R0NHXW) talc (UNII: 7SEV7J4R1U) tocopherol (UNII: R0ZB2556P8) sea salt (UNII: 87GE52P74G) butylated hydroxytoluene (UNII: 1P9D0Z171K) sodium metabisulfite (UNII: 4VON5FNS3C) turmeric (UNII: 856YO1Z64F) ferric oxide red (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-717-01 1 in 1 BOX 1 45 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/30/2014 BUXOM SHOW SOME SKIN WEIGHTLESS FOUNDATION BROAD SPECTRUM SPF 30 CARAMEL KISSES
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-718 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 74 mg in 1 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 61 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) dimethicone (UNII: 92RU3N3Y1O) butylene glycol (UNII: 3XUS85K0RA) diethylhexyl succinate (UNII: 69W9UMG3P8) glycerin (UNII: PDC6A3C0OX) nylon-12 (UNII: 446U8J075B) propanediol (UNII: 5965N8W85T) cyclomethicone (UNII: NMQ347994Z) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) aluminum hydroxide (UNII: 5QB0T2IUN0) diphenylsiloxy phenyl trimethicone (UNII: I445L28B12) disteardimonium hectorite (UNII: X687XDK09L) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) pentylene glycol (UNII: 50C1307PZG) edetate trisodium (UNII: 420IP921MB) aluminum distearate (UNII: 7P1HP1B9UI) tamarind seed (UNII: 6AHP8A7OML) ascorbyl glucoside (UNII: 2V52R0NHXW) talc (UNII: 7SEV7J4R1U) tocopherol (UNII: R0ZB2556P8) sea salt (UNII: 87GE52P74G) butylated hydroxytoluene (UNII: 1P9D0Z171K) sodium metabisulfite (UNII: 4VON5FNS3C) turmeric (UNII: 856YO1Z64F) ferric oxide red (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-718-01 1 in 1 BOX 1 45 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/30/2014 BUXOM SHOW SOME SKIN WEIGHTLESS FOUNDATION BROAD SPECTRUM SPF 30 IN THE DARK
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-719 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 74 mg in 1 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 61 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) dimethicone (UNII: 92RU3N3Y1O) butylene glycol (UNII: 3XUS85K0RA) diethylhexyl succinate (UNII: 69W9UMG3P8) glycerin (UNII: PDC6A3C0OX) nylon-12 (UNII: 446U8J075B) propanediol (UNII: 5965N8W85T) cyclomethicone (UNII: NMQ347994Z) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) aluminum hydroxide (UNII: 5QB0T2IUN0) diphenylsiloxy phenyl trimethicone (UNII: I445L28B12) disteardimonium hectorite (UNII: X687XDK09L) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) pentylene glycol (UNII: 50C1307PZG) edetate trisodium (UNII: 420IP921MB) aluminum distearate (UNII: 7P1HP1B9UI) tamarind seed (UNII: 6AHP8A7OML) ascorbyl glucoside (UNII: 2V52R0NHXW) talc (UNII: 7SEV7J4R1U) tocopherol (UNII: R0ZB2556P8) sea salt (UNII: 87GE52P74G) butylated hydroxytoluene (UNII: 1P9D0Z171K) sodium metabisulfite (UNII: 4VON5FNS3C) turmeric (UNII: 856YO1Z64F) ferric oxide red (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-719-01 1 in 1 BOX 1 45 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/30/2014 BUXOM SHOW SOME SKIN WEIGHTLESS FOUNDATION BROAD SPECTRUM SPF 30 SILKY NEGLI-BEIGE
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-762 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 74 mg in 1 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 61 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) dimethicone (UNII: 92RU3N3Y1O) butylene glycol (UNII: 3XUS85K0RA) diethylhexyl succinate (UNII: 69W9UMG3P8) glycerin (UNII: PDC6A3C0OX) nylon-12 (UNII: 446U8J075B) propanediol (UNII: 5965N8W85T) cyclomethicone (UNII: NMQ347994Z) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) aluminum hydroxide (UNII: 5QB0T2IUN0) diphenylsiloxy phenyl trimethicone (UNII: I445L28B12) disteardimonium hectorite (UNII: X687XDK09L) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) pentylene glycol (UNII: 50C1307PZG) edetate trisodium (UNII: 420IP921MB) aluminum distearate (UNII: 7P1HP1B9UI) tamarind seed (UNII: 6AHP8A7OML) ascorbyl glucoside (UNII: 2V52R0NHXW) talc (UNII: 7SEV7J4R1U) tocopherol (UNII: R0ZB2556P8) sea salt (UNII: 87GE52P74G) butylated hydroxytoluene (UNII: 1P9D0Z171K) sodium metabisulfite (UNII: 4VON5FNS3C) turmeric (UNII: 856YO1Z64F) ferric oxide red (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-762-01 1 in 1 BOX 1 45 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 06/01/2015 BUXOM SHOW SOME SKIN WEIGHTLESS FOUNDATION BROAD SPECTRUM SPF 30 TICKLE THE IVORY
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-763 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 74 mg in 1 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 61 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) dimethicone (UNII: 92RU3N3Y1O) butylene glycol (UNII: 3XUS85K0RA) diethylhexyl succinate (UNII: 69W9UMG3P8) glycerin (UNII: PDC6A3C0OX) nylon-12 (UNII: 446U8J075B) propanediol (UNII: 5965N8W85T) cyclomethicone (UNII: NMQ347994Z) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) aluminum hydroxide (UNII: 5QB0T2IUN0) diphenylsiloxy phenyl trimethicone (UNII: I445L28B12) disteardimonium hectorite (UNII: X687XDK09L) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) pentylene glycol (UNII: 50C1307PZG) edetate trisodium (UNII: 420IP921MB) aluminum distearate (UNII: 7P1HP1B9UI) tamarind seed (UNII: 6AHP8A7OML) ascorbyl glucoside (UNII: 2V52R0NHXW) talc (UNII: 7SEV7J4R1U) tocopherol (UNII: R0ZB2556P8) sea salt (UNII: 87GE52P74G) butylated hydroxytoluene (UNII: 1P9D0Z171K) sodium metabisulfite (UNII: 4VON5FNS3C) turmeric (UNII: 856YO1Z64F) ferric oxide red (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-763-01 1 in 1 BOX 1 45 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 06/01/2015 Labeler - Bare Escentuals Beauty, Inc. (087008363)