| NDC | 0113-7951-81 |
| Set ID | 8e8451d2-33e0-4d56-8fc2-00ad2d2e4511 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | L. Perrigo Company |
| Generic Name | |
| Product Class | alpha-1 Adrenergic Agonist |
| Product Number | |
| Application Number | PART341 |
- Active ingredients (in each caplet) - DAY TIME Severe Cold
- Active ingredients (in each caplet) – NIGHT TIME Cold & Flu
- Purposes - DAY TIME Severe Cold
- Purposes - NIGHT TIME Cold & Flu
-
Uses
- •
- temporarily relieves these common cold and flu symptoms:
- •
- nasal congestion
- •
- cough (DAY TIME Severe Cold only)
- •
- minor aches and pains
- •
- headache
- •
- sore throat
- •
- runny nose and sneezing (NIGHT TIME Cold & Flu only)
- •
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive (DAY TIME Severe Cold only)
- •
- temporarily reduces fever
-
Warnings
Liver warning: These products contain acetaminophen. Severe liver damage may occur if you take
- •
- more than 4,000 mg of acetaminophen in 24 hours
- •
- with other drugs containing acetaminophen
- •
- 3 or more alcoholic drinks every day while using these products
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- •
- skin reddening
- •
- blisters
- •
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
-
Do not use
- •
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- •
- with any other product containing diphenhydramine, even one used on skin (NIGHT TIME Cold & Flu only)
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking these products.
- •
- if you have ever had an allergic reaction to these products or any of their ingredients
-
Ask a doctor before use if you have
- •
- liver disease
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to an enlarged prostate gland
- •
- glaucoma (NIGHT TIME Cold & Flu only)
- •
- a breathing problem such as emphysema or chronic bronchitis (NIGHT TIME Cold & Flu only)
- •
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema (DAY TIME Severe Cold only)
- •
- cough that occurs with too much phlegm (mucus) (DAY TIME Severe Cold only)
- Ask a doctor or pharmacist before use if you are
-
When using these products
- •
- do not use more than directed
- •
- excitability may occur, especially in children (NIGHT TIME Cold & Flu only)
- •
- marked drowsiness may occur (NIGHT TIME Cold & Flu only)
- •
- alcohol, sedatives, and tranquilizers may increase drowsiness (NIGHT TIME Cold & Flu only)
- •
- avoid alcoholic drinks (NIGHT TIME Cold & Flu only)
- •
- be careful when driving a motor vehicle or operating machinery (NIGHT TIME Cold & Flu only)
-
Stop use and ask a doctor if
- •
- nervousness, dizziness, or sleeplessness occur
- •
- pain, nasal congestion or cough gets worse or lasts more than 7 days
- •
- fever gets worse or lasts more than 3 days
- •
- redness or swelling is present
- •
- new symptoms occur
- •
- cough comes back or occurs with rash or headache that lasts. These could be signs of a serious condition. (DAY TIME Severe Cold only)
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients - DAY TIME Severe Cold
- Inactive ingredients – NIGHT TIME Cold & Flu
- Questions or comments?
-
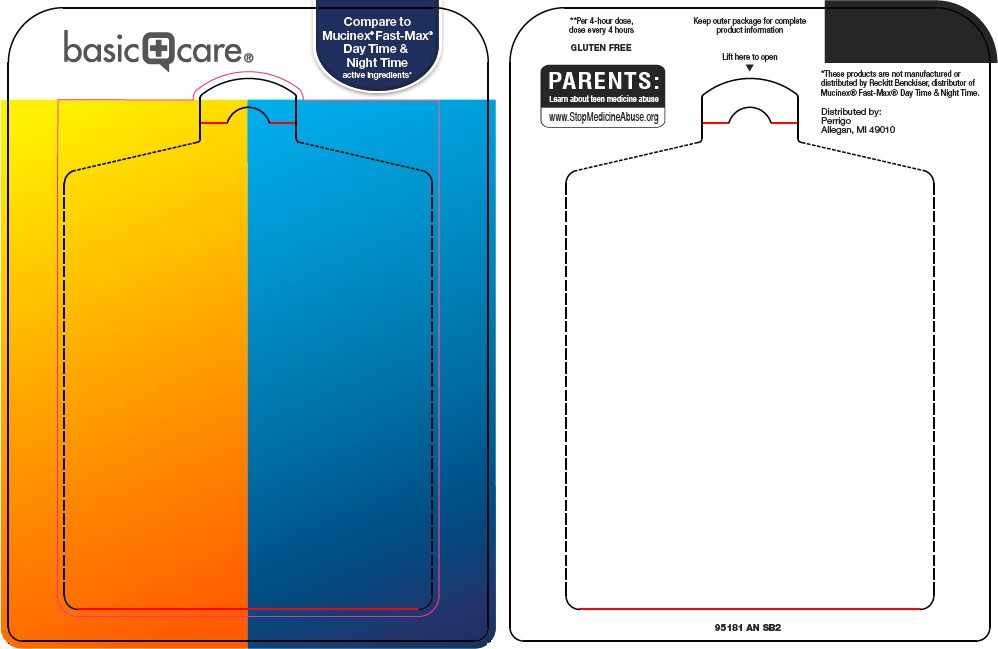
Package/Label Principal Display Panel
Compare to Mucinex® Fast-Max® Day Time & Night Time active ingredients
Maximum Strength
For Ages 12+
daytime severe cold
Acetaminophen
Dextromethorphan HBr
Guaifenesin
Phenylephrine HCl
Pain Reliever
Fever Reducer
Cough Suppressant
Expectorant
Nasal Decongestant
Relieves Aches, Fever & Sore Throat
Controls Cough
Relieves Nasal & Chest Congestion
Thins & Loosens Mucus
actual size
20 CAPLETS
For Ages 12+
night time cold & flu
Acetaminophen
Diphenhydramine HCl
Phenylephrine HCl
Pain Reliever
Fever Reducer
Antihistamine
Nasal Decongestant
Relieves Aches, Fever & Sore Throat
Relieves Nasal Congestion
Relieves Runny Nose & Sneezing
actual size
10 CAPLETS
-
INGREDIENTS AND APPEARANCE
BASIC CARE DAYTIME SEVERE COLD NIGHTTIME COLD AND FLU
acetaminophen, dextromethorphan hydrobromide, guaifenesin, phenylephrine hydrochloride, diphenhydramine hydrochloride kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0113-7951 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0113-7951-81 1 in 1 KIT; Type 0: Not a Combination Product 01/24/2019 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 BLISTER PACK 20 Part 2 5 BLISTER PACK 10 Part 1 of 2 BASIC CARE DAY TIME SEVERE COLD
acetaminophen, dextromethorphan hydrobromide, guaifenesin, phenylephrine hydrochloride tablet, film coatedProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color RED Score no score Shape OVAL Size 20mm Flavor Imprint Code L922 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 10 in 1 CARTON 1 2 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 Part 2 of 2 BASIC CARE NIGHT TIME COLD AND FLU
acetaminophen, diphenhydramine hydrochloride, phenylephrine hydrochloride tablet, film coatedProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color BLUE Score no score Shape OVAL Size 16mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 5 in 1 CARTON 1 2 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 01/24/2019 Labeler - L. Perrigo Company (006013346)