| NDC | 57955-0117-2 |
| Set ID | 258bf062-0e8c-4a4b-b299-b494b7939b66 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | King Bio Inc. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
ACTIVE INGREDIENT
Drug Facts__________________________________________________________________________________________________________
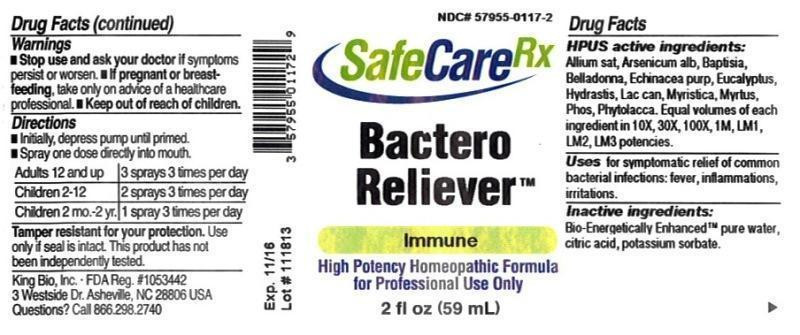
HPUS active ingredients: Allium sativum, Arsenicum album, Baptisia tinctoria, Belladonna, Echinacea purpurea, Eucalyptus globulus, Hydrastis canadensis, Lac caninum, Myristica sebifera, Myrtus communis, Phosphorus, Phytolacca decandra. Equal volumes of each ingredient in 10X, 30X, 100X, 1M, LM1, LM2, LM3 potencies.
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BACTERO RELIEVER
allium sativum, arsenicum album, baptisia tinctoria, belladonna, echinacea purpurea, eucalyptus globulus, hydrastis canadensis, lac caninum, myristica sebifera, myrtus communis, phosphorus, phytolacca decandra liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57955-0117 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GARLIC (UNII: V1V998DC17) (GARLIC - UNII:V1V998DC17) GARLIC 10 [hp_X] in 59 mL ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 10 [hp_X] in 59 mL BAPTISIA TINCTORIA ROOT (UNII: 5EF0HWI5WU) (BAPTISIA TINCTORIA ROOT - UNII:5EF0HWI5WU) BAPTISIA TINCTORIA ROOT 10 [hp_X] in 59 mL ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 10 [hp_X] in 59 mL ECHINACEA PURPUREA (UNII: QI7G114Y98) (ECHINACEA PURPUREA - UNII:QI7G114Y98) ECHINACEA PURPUREA 10 [hp_X] in 59 mL EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) (EUCALYPTUS GLOBULUS LEAF - UNII:S546YLW6E6) EUCALYPTUS GLOBULUS LEAF 10 [hp_X] in 59 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 10 [hp_X] in 59 mL CANIS LUPUS FAMILIARIS MILK (UNII: G39P120JQT) (CANIS LUPUS FAMILIARIS MILK - UNII:G39P120JQT) CANIS LUPUS FAMILIARIS MILK 10 [hp_X] in 59 mL VIROLA SEBIFERA RESIN (UNII: GHJ5XX5SGS) (VIROLA SEBIFERA RESIN - UNII:GHJ5XX5SGS) VIROLA SEBIFERA RESIN 10 [hp_X] in 59 mL MYRTUS COMMUNIS TOP (UNII: 367E55FXGW) (MYRTUS COMMUNIS TOP - UNII:367E55FXGW) MYRTUS COMMUNIS TOP 10 [hp_X] in 59 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 10 [hp_X] in 59 mL PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 10 [hp_X] in 59 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57955-0117-2 59 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/06/2012 Labeler - King Bio Inc. (617901350) Registrant - King Bio Inc. (617901350) Establishment Name Address ID/FEI Business Operations King Bio Inc. 617901350 manufacture(57955-0117)