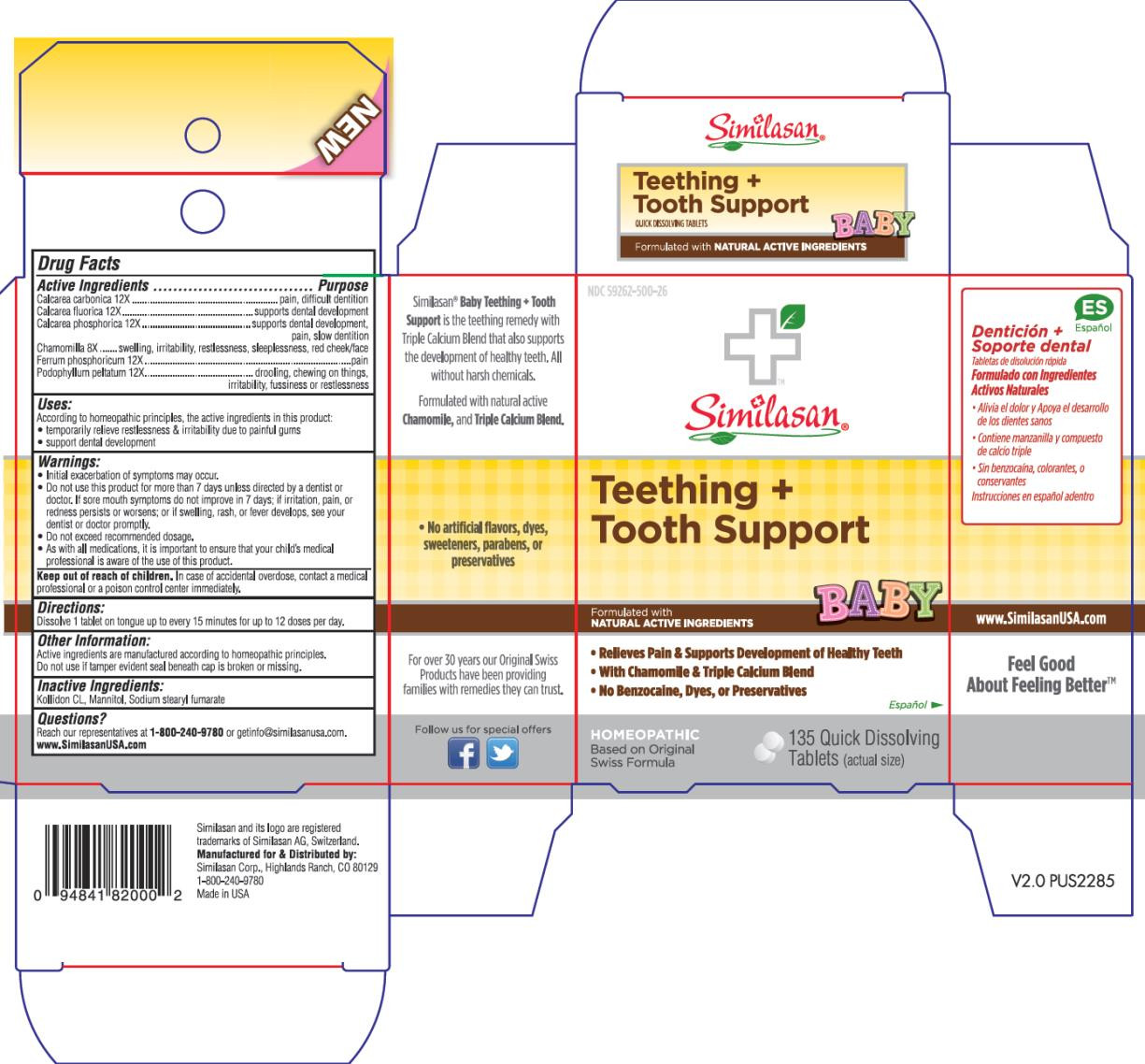
| NDC | 59262-500-26 |
| Set ID | f546e13d-e535-49ba-9169-cfc4ee6a6775 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Similasan Corporation |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Uses:
-
Warnings:
- Initial exacerbation of symptoms may occur.
- Do not use this product for more than 7 days unless directed by a dentist or doctor. If sore mouth symptoms do not improve in 7 days; if irritation, pain, or redness persists or worsens; or if swelling, rash, or fever develops, see your dentist or doctor promptly.
- Do not exceed recommended dosage.
- As with all medications, it is important to ensure that your child’s medical professional is aware of the use of this product.
- Initial exacerbation of symptoms may occur.
- Keep out of reach of children.
- Directions:
- Other Information:
- Inactive Ingredients:
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BABY TEETHING PLUS TOOTH SUPPORT
oyster shell calcium carbonate, crude, calcium fluoride, tribasic calcium phosphate, matricaria recutita, ferrosoferric phosphate, and podophyllum tablet, orally disintegratingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59262-500 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 12 [hp_X] CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 12 [hp_X] TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 12 [hp_X] MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 12 [hp_X] FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERROSOFERRIC PHOSPHATE 12 [hp_X] PODOPHYLLUM (UNII: 2S713A4VP3) (PODOPHYLLUM - UNII:2S713A4VP3) PODOPHYLLUM 12 [hp_X] Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) Product Characteristics Color WHITE Score no score Shape ROUND Size 4mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59262-500-26 1 in 1 BOX 1 135 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 01/01/2015 Labeler - Similasan Corporation (111566530) Establishment Name Address ID/FEI Business Operations Similasan Corporation 111566530 MANUFACTURE(59262-500)