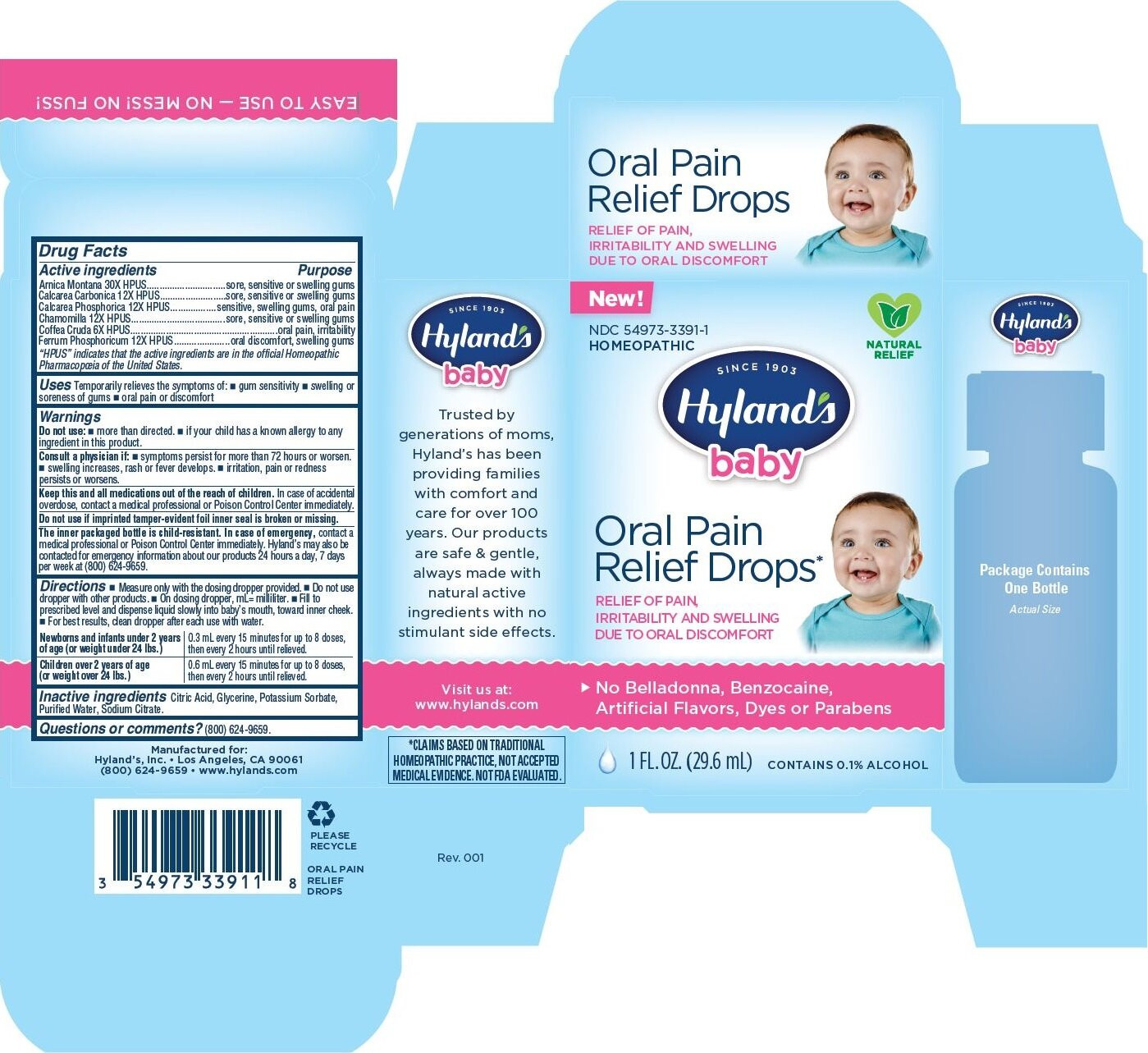
| NDC | 54973-3391-1 |
| Set ID | 7ffe2d76-254a-1406-e053-2991aa0afb61 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Hyland's |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
Drug Facts
Active ingredients
Arnica Montana 30X HPUS
Calcarea Carbonica 12X HPUS
Calcarea Phosphorica 12X HPUS
Chamomilla 12X HPUS
Coffea Cruda 6X HPUS
Ferrum Phosphoricum 12X HPUS
“HPUS” indicates that the active ingredients are in the official Homeopathic
Pharmacopœia of the United States.
Purpose
Arnica Montana 30X HPUS...............................sore, sensitive or swelling gums
Calcarea Carbonica 12X HPUS..........................sore, sensitive or swelling gums
Calcarea Phosphorica 12X HPUS..................sensitive, swelling gums, oral pain
Chamomilla 12X HPUS.....................................sore, sensitive or swelling gums
Coffea Cruda 6X HPUS..........................................................oral pain, irritability
Ferrum Phosphoricum 12X HPUS......................oral discomfort, swelling gums
- Uses
-
Warnings
Consult a physician if
- symptoms persist for more than 72 hours or worsen.
- swelling increases, rash or fever develops.
- irritation, pain or redness persists or worsens.
-
Directions
- Measure only with the dosing dropper provided.
- Do not use dropper with other products.
- On dosing dropper, mL= milliliter.
- Fill to prescribed level and dispense liquid slowly into baby’s mouth, toward inner cheek.
- For best results, clean dropper after each use with water.
Newborns and infants under 2 years
of age (or weight under 24 lbs.)
0.3 mL every 15 minutes for up to 8 doses,
then every 2 hours until relieved.
Children over 2 years of age
(or weight over 24 lbs.)
0.6 mL every 15 minutes for up to 8 doses,
then every 2 hours until relieved.
- Inactive ingredients
- Questions or comments?
- Principal Display Panel - 1 FL. OZ. (29.6 mL) Bottle Carton
-
INGREDIENTS AND APPEARANCE
BABY ORAL PAIN RELIEF DROPS
ferrosoferric phosphate,tribasic calcium phosphate,oyster shell calcium carbonate,crude,arnica montana,arabica coffee bean and matricaria chamomilla liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54973-3391 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 30 [hp_X] in 1 mL OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 12 [hp_X] in 1 mL TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) (PHOSPHATE ION - UNII:NK08V8K8HR) TRIBASIC CALCIUM PHOSPHATE 12 [hp_X] in 1 mL FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERROSOFERRIC PHOSPHATE 12 [hp_X] in 1 mL ARABICA COFFEE BEAN (UNII: 3SW678MX72) (ARABICA COFFEE BEAN - UNII:3SW678MX72) ARABICA COFFEE BEAN 6 [hp_X] in 1 mL MATRICARIA CHAMOMILLA (UNII: G0R4UBI2ZZ) (MATRICARIA CHAMOMILLA - UNII:G0R4UBI2ZZ) MATRICARIA CHAMOMILLA 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54973-3391-1 1 in 1 CARTON 01/24/2019 1 29.6 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/24/2019 Labeler - Hyland's (028570695) Establishment Name Address ID/FEI Business Operations Standard Homeopathic Company 008316655 manufacture(54973-3391) , pack(54973-3391) , label(54973-3391) Establishment Name Address ID/FEI Business Operations Applied Laboratories, Inc. 876754375 manufacture(54973-3391) , pack(54973-3391) , label(54973-3391)