| NDC | 54973-3284-1, 54973-3371-2, 54973-3374-1 |
| Set ID | 7979fc6f-6d2c-56c9-e053-2991aa0ac1f3 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Hyland's |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
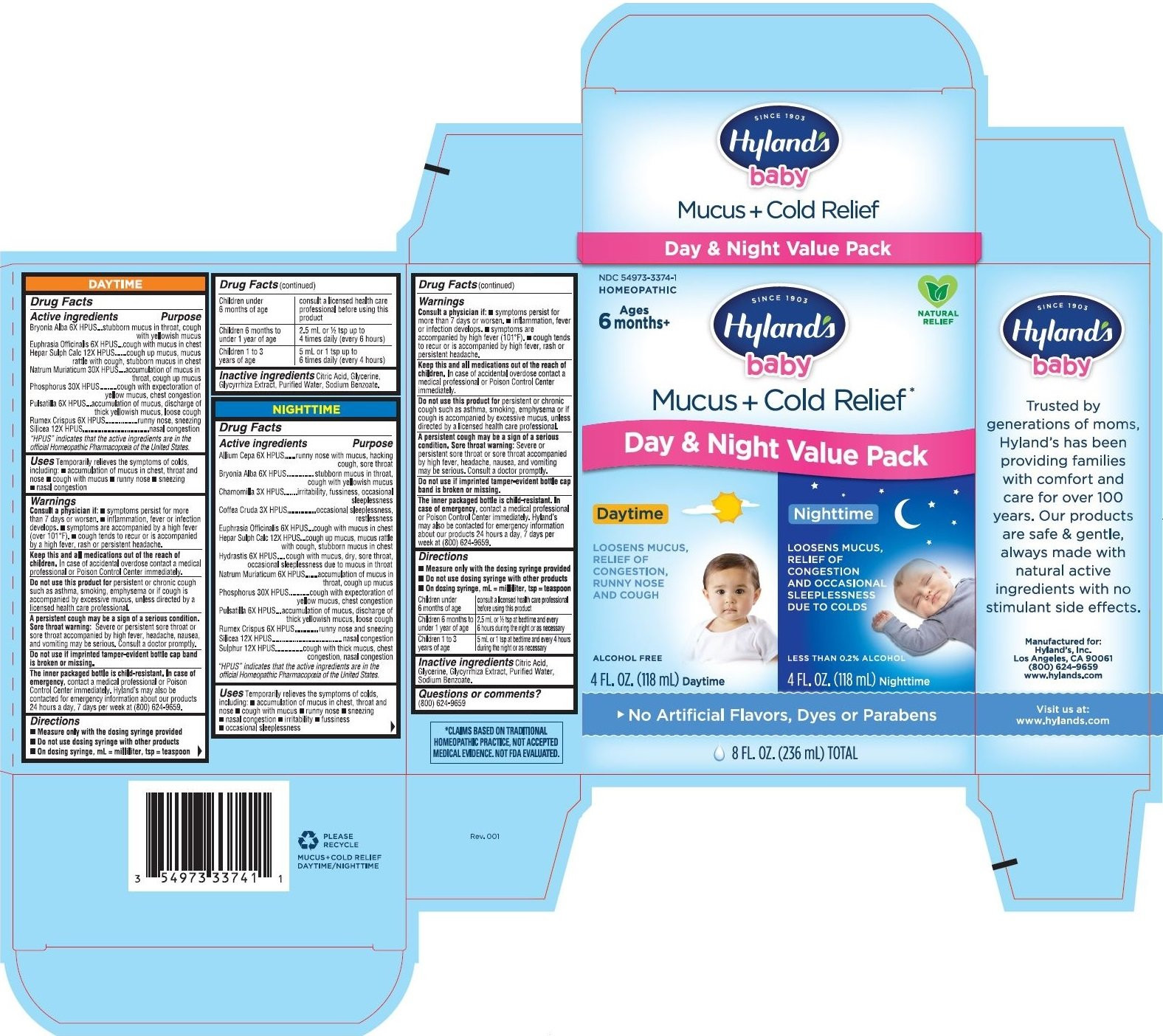
DAYTIME
Drug Facts
Active ingredients
Bryonia Alba 6X HPUS
Euphrasia Officinalis 6X HPUS
Hepar Sulph Calc 12X HPUS
Natrum Muriaticum 30X HPUS
Phosphorus 30X HPUS
Pulsatilla 6X HPUS
Rumex Crispus 6X HPUS
Silicea 12X HPUS
“HPUS” indicates that the active ingredients are in the
official Homeopathic Pharmacopœia of the United States.
Purpose
Bryonia Alba 6X HPUS...stubborn mucus in throat, cough with yellowish mucus
Euphrasia Officinalis 6X HPUS...cough with mucus in chest
Hepar Sulph Calc 12X HPUS......cough up mucus, mucus rattle with cough, stubborn mucus in chest
Natrum Muriaticum 30X HPUS....accumulation of mucus in throat, cough up mucus
Phosphorus 30X HPUS.........cough with expectoration of yellow mucus, chest congestion
Pulsatilla 6X HPUS...accumulation of mucus, discharge of thick yellowish mucus, loose cough
Rumex Crispus 6X HPUS.................runny nose, sneezing
Silicea 12X HPUS...................................nasal congestion
Uses
Temporarily relieves the symptoms of colds, including: • accumulation of mucus in chest, throat and nose • cough with mucus
• runny nose • sneezing • nasal congestion
Warnings
Consult a physician if
• symptoms persist for more than 7 days or worsen. • inflammation, fever or infection develops. • symptoms are accompanied by a high fever (over 101°F). • cough tends to recur or is accompanied by a high fever, rash or persistent headache.
Keep this and all medications out of the reach of children
In case of accidental overdose contact a medical professional or Poison Control Center immediately.
Do not use this product for
persistent or chronic cough such as asthma, smoking, emphysema or if cough is accompanied by excessive mucus, unless directed by a licensed health care professional.
Directions
• Measure only with the dosing syringe provided
• Do not use dosing syringe with other products
• On dosing syringe, mL = milliliter, tsp = teaspoon
-
NIGHTTIME
Active ingredients
Allium Cepa 6X HPUS
Bryonia Alba 6X HPUS
Chamomilla 3X HPUS
Coffea Cruda 3X HPUS
Euphrasia Officinalis 6X HPUS
Hepar Sulph Calc 12X HPUS
Hydrastis 6X HPUS
Natrum Muriaticum 6X HPUS
Phosphorus 30X HPUS
Pulsatilla 6X HPUS
Rumex Crispus 6X HPUS
Silicea 12X HPUS
Sulphur 12X HPUS
“HPUS” indicates that the active ingredients are in the
official Homeopathic Pharmacopœia of the United States.
Purpose
Allium Cepa 6X HPUS......runny nose with mucus, hacking cough, sore throat
Bryonia Alba 6X HPUS.............. stubborn mucus in throat, cough with yellowish mucus
Chamomilla 3X HPUS.......irritability, fussiness, occasional sleeplessness
Coffea Cruda 3X HPUS...............occasional sleeplessness, restlessness
Euphrasia Officinalis 6X HPUS...cough with mucus in chest
Hepar Sulph Calc 12X HPUS...cough up mucus, mucus rattle with cough, stubborn mucus in chest
Hydrastis 6X HPUS.... cough with mucus, dry, sore throat, occasional sleeplessness due to mucus in throat
Natrum Muriaticum 6X HPUS.......accumulation of mucus in throat, cough up mucus
Phosphorus 30X HPUS...........cough with expectoration of yellow mucus, chest congestion
Pulsatilla 6X HPUS...accumulation of mucus, discharge of thick yellowish mucus, loose cough
Rumex Crispus 6X HPUS............runny nose and sneezing
Silicea 12X HPUS.....................................nasal congestion
Sulphur 12X HPUS...............cough with thick mucus, chest congestion, nasal congestion
Uses
Temporarily relieves the symptoms of colds,including: • accumulation of mucus in chest, throat and nose • cough with mucus
• runny nose • sneezing •nasal congestion • irritability • fussiness • occasional sleeplessness
Drug Facts (continued)
Warnings
Consult a physician if:
• symptoms persist for more than 7 days or worsen. • inflammation, fever or infection develops. • symptoms are accompanied by high fever (101°F). • cough tends to recur or is accompanied by high fever, rash or persistent headache.
Keep this and all medications out of the reach of children.
In case of accidental overdose contact a medical professional or Poison Control Center immediately.
Do not use this product for
persistent or chronic cough such as asthma, smoking, emphysema or if cough is accompanied by excessive mucus, unles directed by a licensed health care professional.
Directions
- Measure only with the dosing syringe provided
- Do not use dosing syringe with other products
- On dosing syringe, mL = milliliter, tsp = teaspoon
Children under
6 months of age
consult a licensed health care professional
before using this product
Children 6 months to
under 1 year of age
2.5 mL or ½ tsp at bedtime and every
6 hours during the night or as necessary
Children 1 to 3
years of age
5 mL or 1 tsp at bedtime and every 4 hours
during the night or as necessary
- SPL UNCLASSIFIED SECTION
-
PRIMARY DISPLAY PANEL - Day & Night Value Pack Kit
NDC 54973-3374-1
HOMEOPATHIC
Ages
6 months+
NATURAL
RELIEF
SINCE 1903
Hyland's
baby
Mucus + Cold Relief*
Day & Night Value Pack
Daytime
LOOSENS MUCUS,
RELIEF OF
CONGESTION,
RUNNY NOSE
AND COUGH
ALCOHOL FREE
4 FL. OZ. (118 mL) Daytime
Nighttime
LOOSENS MUCUS,
RELIEF OF
CONGESTION
AND OCCASIONAL
SLEEPLESSNESS
DUE TO COLDS
LESS THAN 0.2% ALCOHOL
4 FL. OZ. (118 mL) Nighttime
No Artificial Flavors, Dyes or Parabens
8 FL. OZ. (236 mL) TOTAL

-
INGREDIENTS AND APPEARANCE
BABY MUCUS PLUS COLD RELIEF DAY AND NIGHT VALUE PACK
calcium sulfide,phosphorus,sulfur,rumex crispus root,sodium chloride,arabica coffee bean,anemone pulsatilla,silicon dioxide, goldenseal,onion,bryonia alba root,euphrasia stricta and matricaria chamomilla kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54973-3374 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54973-3374-1 1 in 1 CARTON; Type 0: Not a Combination Product 11/01/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PLASTIC 118 mL Part 2 1 BOTTLE, PLASTIC 118 mL Part 1 of 2 BABY NIGHTTIME MUCUS PLUS COLD RELIEF
calcium sulfide,phosphorus,sulfur,rumex crispus root,sodium chloride,arabica coffee bean,anemone pulsatilla,silicon dioxide, goldenseal,onion,bryonia alba root,euphrasia stricta and matricaria chamomilla liquidProduct Information Item Code (Source) NDC:54973-3371 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 12 [hp_X] in 1 mL ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 6 [hp_X] in 1 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 30 [hp_X] in 1 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 6 [hp_X] in 1 mL MATRICARIA CHAMOMILLA (UNII: G0R4UBI2ZZ) (MATRICARIA CHAMOMILLA - UNII:G0R4UBI2ZZ) MATRICARIA CHAMOMILLA 3 [hp_X] in 1 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 12 [hp_X] in 1 mL RUMEX CRISPUS ROOT (UNII: 9N1RM2S62C) (RUMEX CRISPUS ROOT - UNII:9N1RM2S62C) RUMEX CRISPUS ROOT 6 [hp_X] in 1 mL ARABICA COFFEE BEAN (UNII: 3SW678MX72) (ARABICA COFFEE BEAN - UNII:3SW678MX72) ARABICA COFFEE BEAN 3 [hp_X] in 1 mL ANEMONE PULSATILLA (UNII: I76KB35JEV) (ANEMONE PULSATILLA - UNII:I76KB35JEV) ANEMONE PULSATILLA 6 [hp_X] in 1 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 12 [hp_X] in 1 mL EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 6 [hp_X] in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 6 [hp_X] in 1 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 6 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) SODIUM BENZOATE (UNII: OJ245FE5EU) WATER (UNII: 059QF0KO0R) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54973-3371-2 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/01/2018 Part 2 of 2 BABY MUCUS AND COLD RELIEF
bryonia alba root, euphrasia stricta, calcium sulfide, sodium chloride, phosphorus, anemone pulsatilla, rumex crispus root, and silicon dioxide liquidProduct Information Item Code (Source) NDC:54973-3284 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 6 [hp_X] in 1 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 12 [hp_X] in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 30 [hp_X] in 1 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 30 [hp_X] in 1 mL ANEMONE PULSATILLA (UNII: I76KB35JEV) (ANEMONE PULSATILLA - UNII:I76KB35JEV) ANEMONE PULSATILLA 6 [hp_X] in 1 mL RUMEX CRISPUS ROOT (UNII: 9N1RM2S62C) (RUMEX CRISPUS ROOT - UNII:9N1RM2S62C) RUMEX CRISPUS ROOT 6 [hp_X] in 1 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 12 [hp_X] in 1 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 6 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM BENZOATE (UNII: OJ245FE5EU) GLYCERIN (UNII: PDC6A3C0OX) LICORICE (UNII: 61ZBX54883) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54973-3284-1 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/13/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/01/2018 Labeler - Hyland's (028570695) Establishment Name Address ID/FEI Business Operations Nexgen Pharma, Inc. 079424083 manufacture(54973-3374) , pack(54973-3374) , label(54973-3374)