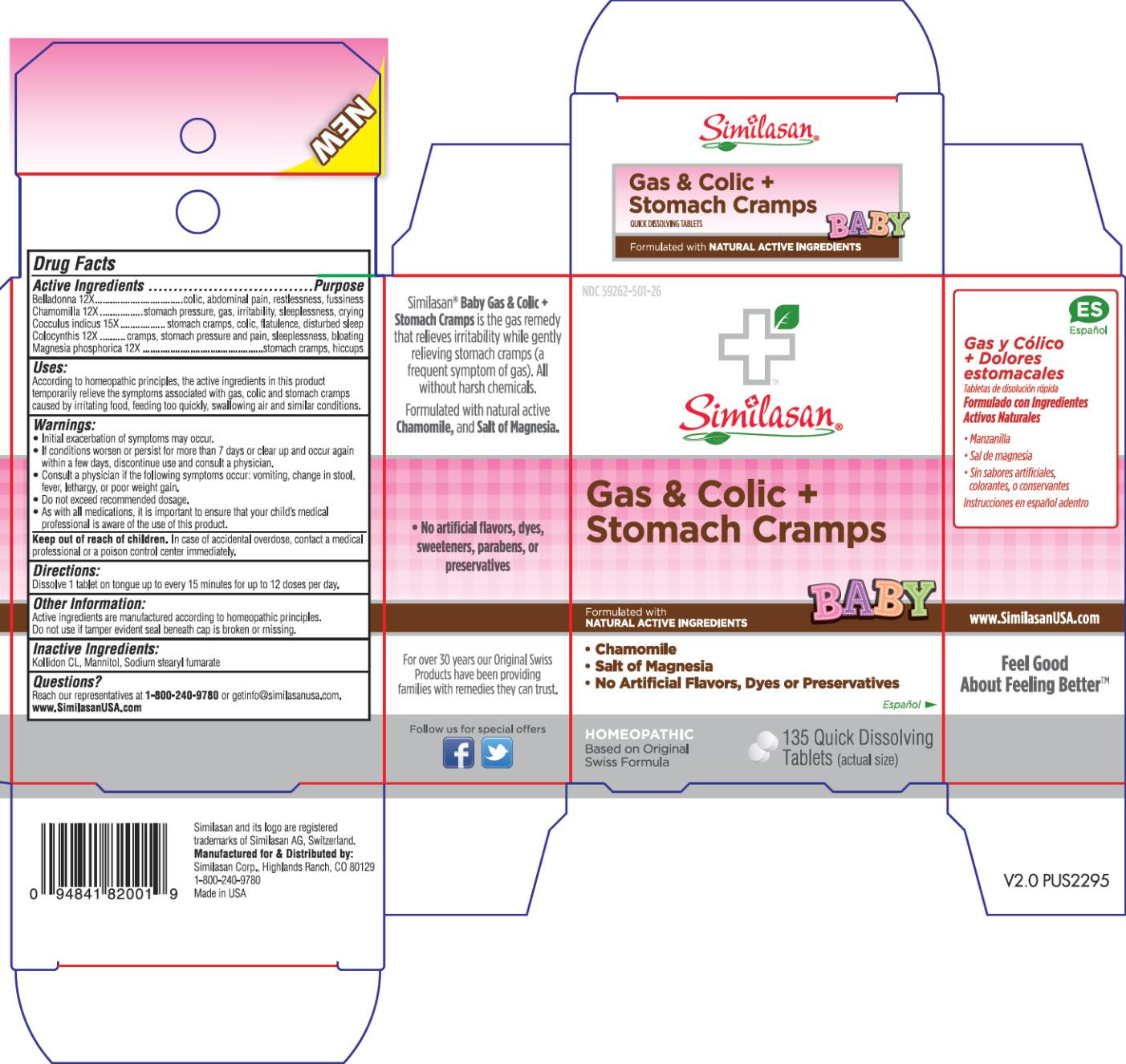
| NDC | 59262-501-26 |
| Set ID | ffcfc152-3232-4a88-9b78-e6981fc87684 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Similasan Corporation |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
-
Uses:
According to homeopathic principles, the active ingredients in this product temporarily relieve the symptoms associated with gas and colic caused by irritating food, feeding too quickly, swallowing air and similar conditions such as:
- gas discomfort
- colic
- stomach cramps
- stomach pressure
- hiccups
- crying
- irritability
- gas discomfort
-
Warnings:
- Initial exacerbation of symptoms may occur.
- If conditions worsen or persist for more than 7 days or clear up and occur again within a few days, discontinue use and consult a physician.
- Consulta a physician if the following symptoms occur: vomiting, change in stool, fever, lethargy, or poor weight gain.
- Do not exceed recommended dosage.
- As with all medications, it is important to ensure that your child’s medical professional is aware of the use of this product.
- Initial exacerbation of symptoms may occur.
- Keep out of reach of children.
- Directions:
- Other Information:
- Inactive Ingredients:
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BABY GAS AND COLIC PLUS STOMACH CRAMPS
atropa belladonna, matricaria recutita, anamirta cocculus whole, citrullus colocynthis fruit pulp and magnesium phosphate, dibasic trihydrate tablet, orally disintegratingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59262-501 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 12 [hp_X] MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 12 [hp_X] ANAMIRTA COCCULUS WHOLE (UNII: 8O4P2U3QO2) (ANAMIRTA COCCULUS WHOLE - UNII:8O4P2U3QO2) ANAMIRTA COCCULUS WHOLE 15 [hp_X] CITRULLUS COLOCYNTHIS FRUIT PULP (UNII: 23H32AOH17) (CITRULLUS COLOCYNTHIS FRUIT PULP - UNII:23H32AOH17) CITRULLUS COLOCYNTHIS FRUIT PULP 12 [hp_X] MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE (UNII: HF539G9L3Q) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE 12 [hp_X] Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) Product Characteristics Color WHITE Score no score Shape ROUND Size 4mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59262-501-26 1 in 1 BOX 1 135 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 01/01/2015 Labeler - Similasan Corporation (111566530) Establishment Name Address ID/FEI Business Operations Similasan Corporation 111566530 MANUFACTURE(59262-501)