| NDC | 42681-9032-1, 42681-9032-2 |
| Set ID | d03ce312-2c79-4cc3-b6bf-3965aac13610 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | WFM Private Label, LP |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number |
-
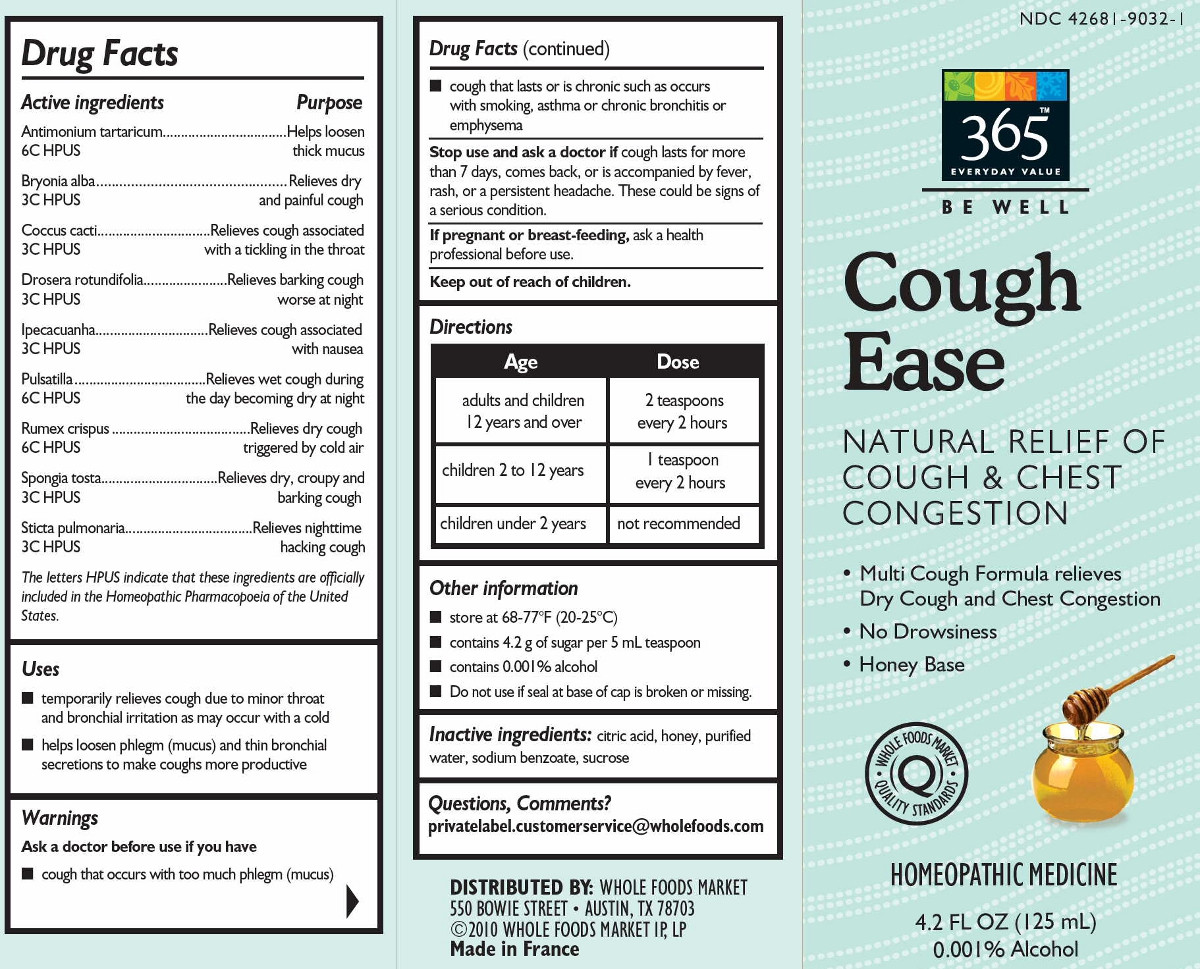
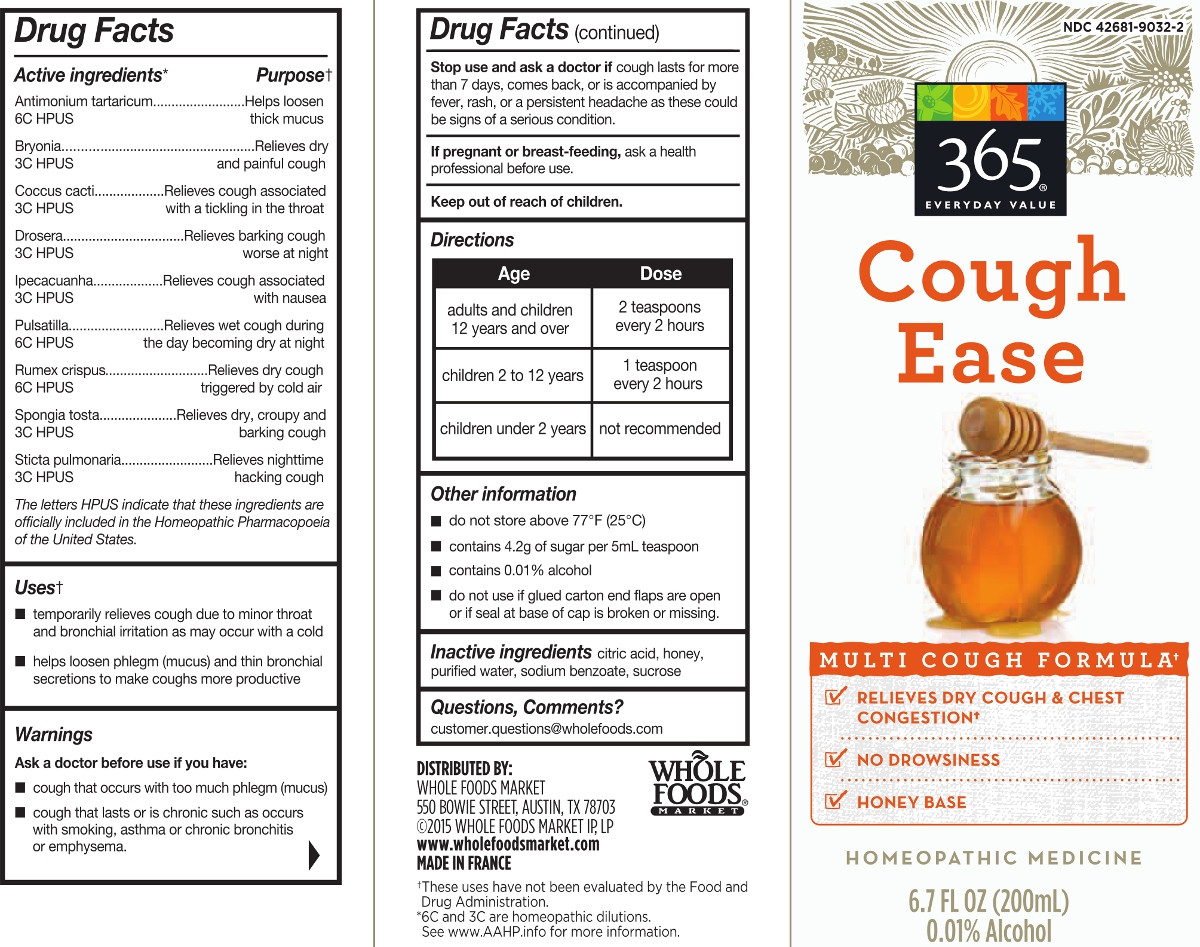
ACTIVE INGREDIENT
Antimonium tartaricum 6C (12.4 mg) ** (contains less an 10 -11 mg antimony)
Bryonia alba 3C (12.4 mg)**
Coccus cacti 3C (12.4 mg)**
Drosera rotundifolia 3C (12.4 mg)**
Ipecacuanha 3C (12.4 mg)** (contains less than 10 -6 mg emetine alkaloids)
Pulsatilla 6C (12.4 mg)**
Rumex crispus 6C (12.4 mg)** (contains less than 10 -13 mg anthraquinone glycosides)
Spongia tosta 3C (12.4 mg)**
Sticta pulmonaria 3C (12.4 mg)**(in each mL)
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
-
PURPOSE
Antimonium tartaricum ... 6C Helps loosen thick mucus*
Bryonia alba 3C ... Relieves dry and painful cough*
Coccus cacti 3C ... Relieves cough associated with a tickling in the throat*
Drosera rotundifolia 3C ... Relieves barking cough worse at night*
Ipecacuanha 3C ... Relieves cough associated with nausea*
Pulsatilla 6C ... Relieves wet cough during the day becoming worse at night*
Rumex crispus 6C ... Relieves dry cough triggered by cold air*
Spongia tosta 3C ... Relieves dry, croupy and barking cough*
Sticta pulmonaria 3C ... Relieves nighttime hacking cough* - INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
SPL UNCLASSIFIED SECTION
do not store above 77° F (25° C)
contains 4 g of sugar per 5 mL teaspoon
dispose of 1 year after opening
do not use if glued carton end flaps are open of if the seal at base of cap is broken or missing
Multi-Symptom Relief
Relieves Dry Cough & Chest Congestion*
No Drowsiness
Made with Honey
Homeopathic Medicine
6.7 FL OZ (200 mL)
Less than 0.01% Alcohol
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE, NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
**6C and 3C are homeopathic dilutions. See www.theAAHP.org for more information. - INACTIVE INGREDIENT
- QUESTIONS
- DRUG INTERACTIONS
- WHEN USING
- Principle Display Panel
-
INGREDIENTS AND APPEARANCE
365 EVERYDAY VALUE BE WELL COUGH EASE
antimony potassium tartrate, bryonia alba root, protortonia cacti, drosera rotundifolia, ipecac, pulsatilla vulgaris, rumex crispus root, pongia officinalis skeleton, roasted, lobaria pulmonaria syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42681-9032 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 3 [hp_C] in 1 mL PROTORTONIA CACTI (UNII: LZB7TFX1LT) (PROTORTONIA CACTI - UNII:LZB7TFX1LT) PROTORTONIA CACTI 3 [hp_C] in 1 mL DROSERA ROTUNDIFOLIA (UNII: QR44N9XPJQ) (DROSERA ROTUNDIFOLIA - UNII:QR44N9XPJQ) DROSERA ROTUNDIFOLIA 3 [hp_C] in 1 mL IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 3 [hp_C] in 1 mL PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 6 [hp_C] in 1 mL RUMEX CRISPUS ROOT (UNII: 9N1RM2S62C) (RUMEX CRISPUS ROOT - UNII:9N1RM2S62C) RUMEX CRISPUS ROOT 6 [hp_C] in 1 mL SPONGIA OFFICINALIS SKELETON, ROASTED (UNII: 1PIP394IID) (SPONGIA OFFICINALIS SKELETON, ROASTED - UNII:1PIP394IID) SPONGIA OFFICINALIS SKELETON, ROASTED 3 [hp_C] in 1 mL LOBARIA PULMONARIA (UNII: D1YM0P5Z2T) (LOBARIA PULMONARIA - UNII:D1YM0P5Z2T) LOBARIA PULMONARIA 3 [hp_C] in 1 mL ANTIMONY POTASSIUM TARTRATE (UNII: DL6OZ476V3) (ANTIMONY CATION (3+) - UNII:069647RPT5) ANTIMONY POTASSIUM TARTRATE 6 [hp_C] in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) HONEY (UNII: Y9H1V576FH) SUCROSE (UNII: C151H8M554) WATER (UNII: 059QF0KO0R) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM BENZOATE (UNII: OJ245FE5EU) Product Characteristics Color Score Shape Size Flavor HONEY (Honey) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42681-9032-1 125 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2011 08/30/2018 2 NDC:42681-9032-2 200 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/03/2011 Labeler - WFM Private Label, LP (196175616)
 ETU.1FGJ 10.2020.jpg)
 ETI.1G6U (bottle).jpg)

 Package images
Package images

